Lipidomic analysis identifies long-chain acylcarnitine as a target for ischemic stroke
- PMID: 37572732
- PMCID: PMC11258661
- DOI: 10.1016/j.jare.2023.08.007
Lipidomic analysis identifies long-chain acylcarnitine as a target for ischemic stroke
Abstract
Introduction: Lipid metabolism dysfunction is widely involved in the pathological process of acute ischemic stroke (AIS). The coordination of lipid metabolism between neurons and astrocytes is of great significance. However, the full scope of lipid dynamic changes and the function of key lipids during AIS remain unknown. Hence, identifying lipid alterations and characterizing their key roles in AIS is of great importance.
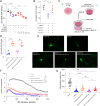
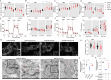
Methods: Untargeted and targeted lipidomic analyses were applied to profile lipid changes in the ischemic penumbra and peripheral blood of transient middle cerebral artery occlusion (tMCAO) mice as well as the peripheral blood of AIS patients. Infarct volume and neurological deficits were assessed after tMCAO. The cell viability and dendritic complexity of primary neurons were evaluated by CCK8 assay and Sholl analysis. Seahorse, MitoTracker Green, tetramethyl rhodamine methyl ester (TMRM), 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) and MitoSOX were used as markers of mitochondrial health. Fluorescent and isotopic free fatty acid (FFA) pulse-chase assays were used to track FFA flux in astrocytes.
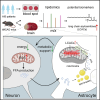
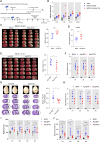
Results: Long-chain acylcarnitines (LCACs) were the lipids with the most dramatic changes in the ischemic penumbra and peripheral blood of tMCAO mice. LCACs were significantly elevated on admission in AIS patients and associated with poor outcomes in AIS patients. Increasing LCACs through a bolus administration of palmitoylcarnitine amplified stroke injury, while decreasing LCACs by overexpressing carnitine palmitoyltransferase 2 (CPT2) ameliorated stroke injury. Palmitoylcarnitine aggravated astrocytic mitochondrial damage after OGD/R, while CPT2 overexpression in astrocytes ameliorated cocultured neuron viability. Further study revealed that astrocytes stimulated by OGD/R liberated FFAs from lipid droplets into mitochondria to form LCACs, resulting in mitochondrial damage and lowered astrocytic metabolic support and thereby aggravated neuronal damage.
Conclusion: LCACs could accumulate and damage neurons by inducing astrocytic mitochondrial dysfunction in AIS. LCACs play a crucial role in the pathology of AIS and are novel promising diagnostic and prognostic biomarkers for AIS.
Keywords: Astrocytic mitochondrial; Biomarker; Ischemic stroke; Lipid droplet; Long-chain acylcarnitine.
Copyright © 2024. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Ma H., et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N Engl J Med. 2019;380:1795–1803. - PubMed
-
- Powers WJ et al., 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. (2018).
-
- M. D, et al., Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation133 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

