GLP-2 Improves Hepatic Inflammation and Fibrosis in Mdr2-/- Mice Via Activation of NR4a1/Nur77 in Hepatic Stellate Cells and Intestinal FXR Signaling
- PMID: 37572734
- PMCID: PMC10522987
- DOI: 10.1016/j.jcmgh.2023.08.003
GLP-2 Improves Hepatic Inflammation and Fibrosis in Mdr2-/- Mice Via Activation of NR4a1/Nur77 in Hepatic Stellate Cells and Intestinal FXR Signaling
Abstract
Background & aims: Glucagon-like peptide (GLP)-2 may exert antifibrotic effects on hepatic stellate cells (HSCs). Thus, we aimed to test whether application of the GLP-2 analogue teduglutide has hepatoprotective and antifibrotic effects in the Mdr2/Abcb4-/- mouse model of sclerosing cholangitis displaying hepatic inflammation and fibrosis.
Methods: Mdr2-/- mice were injected daily for 4 weeks with teduglutide followed by gene expression profiling (bulk liver; isolated HSCs) and immunohistochemistry. Activated HSCs (LX2 cells) and immortalized human hepatocytes and human intestinal organoids were treated with GLP-2. mRNA profiling by reverse transcription polymerase chain reaction and electrophoretic mobility shift assay using cytosolic and nuclear protein extracts was performed.
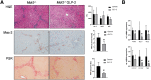
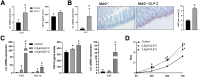
Results: Hepatic inflammation, fibrosis, and reactive cholangiocyte phenotype were improved in GLP-2-treated Mdr2-/- mice. Primary HSCs isolated from Mdr2-/- mice and LX2 cells exposed to GLP-2 in vitro displayed significantly increased mRNA expression levels of NR4a1/Nur77 (P < .05). Electrophoretic mobility shift assay revealed an increased nuclear NR4a1 binding after GLP-2 treatment in LX2 cells. Moreover, GLP-2 alleviated the Tgfβ-mediated reduction of NR4a1 nuclear binding activity. In vivo, GLP-2 treatment of Mdr2-/- mice resulted in increased intrahepatic levels of muricholic acids (accordingly Cyp2c70 mRNA expression was significantly increased), and in reduced mRNA levels of Cyp7a1 and FXR. Serum Fgf15 levels were increased in Mdr2-/- mice treated with GLP-2. Accordingly, GLP-2 treatment of human intestinal organoids activated their FXR-FGF19 signaling axis.
Conclusions: GLP-2 treatment increased NR4a1/Nur77 activation in HSCs, subsequently attenuating their activation. GLP-2 promoted intestinal Fxr-Fgf15/19 signaling resulting in reduced Cyp7a1 and increased Cyp2c70 expression in the liver, contributing to hepatoprotective and antifibrotic effects of GLP-2 in the Mdr2-/- mouse model.
Keywords: Bile Acid Homeostasis; FGF15/19; Fibrosis; Nuclear Binding.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


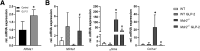


References
-
- Fickert P., Fuchsbichler A., Wagner M., et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. - PubMed
-
- Lazaridis K.N., Strazzabosco M., Larusso N.F. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. - PubMed
-
- Nishio T., Hu R., Koyama Y., et al. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J Hepatol. 2019;71:573–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

