The Normal Range of Baseline Tryptase Should Be 1 to 15 ng/mL and Covers Healthy Individuals With HαT
- PMID: 37572755
- PMCID: PMC11869997
- DOI: 10.1016/j.jaip.2023.08.008
The Normal Range of Baseline Tryptase Should Be 1 to 15 ng/mL and Covers Healthy Individuals With HαT
Abstract
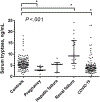
Physiological levels of basal serum tryptase vary among healthy individuals, depending on the numbers of mast cells, basal secretion rate, copy numbers of the TPSAB1 gene encoding alpha tryptase, and renal function. Recently, there has been a growing debate about the normal range of tryptase because individuals with the hereditary alpha tryptasemia (HαT) trait may or may not be symptomatic, and if symptomatic, uncertainty exists as to whether this trait directly causes clinical phenotypes or aggravates certain conditions. In fact, most HαT-positive cases are regarded as asymptomatic concerning mast cell activation. To address this point, experts of the European Competence Network on Mastocytosis (ECNM) and the American Initiative in Mast Cell Diseases met at the 2022 Annual ECNM meeting and discussed the physiological tryptase range. Based on this discussion, our faculty concluded that the normal serum tryptase range should be defined in asymptomatic controls, inclusive of individuals with HαT, and based on 2 SDs covering the 95% confidence interval. By applying this definition in a literature screen, the normal basal tryptase in asymptomatic controls (HαT-positive persons included) ranges between 1 and 15 ng/mL. This definition should avoid overinterpretation, unnecessary referrals, and unnecessary anxiety or anticipatory fear of illness in healthy individuals.
Keywords: Hereditary alpha tryptasemia; Mast cell activation syndromes; Mastocytosis; Tryptase.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
P. Valent has a research grant from AOP Orphan; and serves on advisory boards and receives honoraria from Novartis, AOP Orphan, BMS, Stemline, Pfizer, Incyte, and Blueprint. G. Hoermann has a consultancy and receives honoraria from Novartis, Incyte, Merck, Sharp, and Dohme (MSD), Jazz Pharmaceuticals, Cogent Biosciences, and Blueprint Medicine. P. Bonadonna has a consultancy and receives honoraria from Novartis, Blueprint, Alk and Abello; and receives support in clinical trials from Blueprint. K. Hartmann has a research grant from Thermo Fisher; and has a consultancy and receives honoraria from ALK, Allergopharma, Blueprint, Cogent, Leo Pharma, Menarini, Novartis, Pfizer, Sanofi, Takeda, and Thermo Fisher. W. R. Sperr has a consultancy and receives honoraria from Thermo Fisher, AbbVie, Novartis, Pfizer, Incyte, Deciphera, Jazz, and Teva; and has a research project from Pfizer. S. Broesby-Olsen receives speaker honoraria from Novartis, and Thermo Fisher; and is a study steering committee member for Blueprint Medicines. K. Brockow receives honoraria from Thermo Fisher, Blueprint, and Novartis. M. Niedoszytko serves on an advisory board and receives honoraria from Thermo Fisher, AB Science, and Novartis. O. Hermine has research grants from Blueprint, Abbvie, and Novartis; and serves on as advisory board and receives honoraria from AB Science. Y. Chantran has research grants from Blueprint Medicines and Thermo Fisher; and serves on advisory boards and receives honoraria from Blueprint Medicines and Thermo Fisher. J. H. Butterfield shares royalty payments with Mayo Clinic for licensing of the HMC-1 cell line to Millipore/Sigma. G. Greiner receives honoraria from Novartis, Pfizer, Roche, and Thermo Fisher Scientific. V. Sabato serves on advisory boards for Blueprint Medicines, Cogent, and Novartis; and receives honoraria from Thermo Fisher. D. H. Radia receives research funding from Blueprint Medicines; and serves on advisory boards, does consulting, receives honoraria from Blueprint Medicines and Cogent Biosciences. F. Siebenhaar receives research funding from Allakos, Blueprint, Celldex, Novartis, and Third Harmonic Bio; and serves on advisory boards, does consulting, and receives honoraria from Allakos, Blueprint, Celldex, Cogent, Granular, Invea, Noucor, Novartis, Moxie, Sanofi/Regeneron, and Third Harmonic Bio. M. Triggiani serves on advisory boards for Blueprint Medicines, and Cogent. I. Alvarez-Twose receives a research grant from Blueprint Medicines; and serves on advisory boards and receives honoraria from Blueprint Medicines and Novartis. K. Sotlar serves on advisory boards and receives honoraria from Novartis, Blueprint Medicines, and AstraZeneca. A. Reiter receives consultancy honoraria from Novartis, Blueprint, and Deciphera; receives research support from Novartis. H.-P. Horny receives consultancy honoraria from Novartis and Blueprint. A. Orfao receives consultancy honoraria from Novartis and Blueprint. S. J. Galli does consulting for Evommune and Jasper Therapeutics, Inc. L. B. Schwartz receives royalties from Thermo Fisher for the tryptase assay that are shared with its inventor, L.B.S. J. Gotlib receives research funding from Incyte, Novartis, Kartos, Blueprint Medicines, Cogent Biosciences, Abbvie, BMS, Protagonist Therapeutics, and Imago Biosciences; and serves on advisory boards, does consulting, and receives honoraria from Incyte, Novartis, Kartos, Blueprint Medicines, Cogent Biosciences, Abbvie, Protagonist Therapeutics, PharmaEssentia, and Imago Biosciences. M. Arock receives a research grant from Blueprint Medicines; and serves on advisory boards and receives honoraria from AB Science, Blueprint Medicines, Novartis, and Thermo Fisher. C. Akin receives research grants from Blueprint and Cogent; and has consultancy agreements with Blueprint, Cogent, and Novartis. The rest of the authors declare that they have no relevant conflicts of interest.
Figures
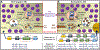


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

