What Can Ribo-Seq, Immunopeptidomics, and Proteomics Tell Us About the Noncanonical Proteome?
- PMID: 37572790
- PMCID: PMC10506109
- DOI: 10.1016/j.mcpro.2023.100631
What Can Ribo-Seq, Immunopeptidomics, and Proteomics Tell Us About the Noncanonical Proteome?
Abstract
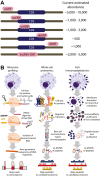
Ribosome profiling (Ribo-Seq) has proven transformative for our understanding of the human genome and proteome by illuminating thousands of noncanonical sites of ribosome translation outside the currently annotated coding sequences (CDSs). A conservative estimate suggests that at least 7000 noncanonical ORFs are translated, which, at first glance, has the potential to expand the number of human protein CDSs by 30%, from ∼19,500 annotated CDSs to over 26,000 annotated CDSs. Yet, additional scrutiny of these ORFs has raised numerous questions about what fraction of them truly produce a protein product and what fraction of those can be understood as proteins according to conventional understanding of the term. Adding further complication is the fact that published estimates of noncanonical ORFs vary widely by around 30-fold, from several thousand to several hundred thousand. The summation of this research has left the genomics and proteomics communities both excited by the prospect of new coding regions in the human genome but searching for guidance on how to proceed. Here, we discuss the current state of noncanonical ORF research, databases, and interpretation, focusing on how to assess whether a given ORF can be said to be "protein coding."
Keywords: Ribo-Seq; immunopeptidomics; mass spectrometry; microprotein; noncanonical ORF.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures




Update of
-
What can Ribo-seq and proteomics tell us about the non-canonical proteome?bioRxiv [Preprint]. 2023 May 18:2023.05.16.541049. doi: 10.1101/2023.05.16.541049. bioRxiv. 2023. Update in: Mol Cell Proteomics. 2023 Sep;22(9):100631. doi: 10.1016/j.mcpro.2023.100631. PMID: 37292611 Free PMC article. Updated. Preprint.
References
-
- Blencowe B.J. The relationship between alternative splicing and proteomic complexity. Trends Biochem. Sci. 2017;42:407–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

