Synergistic efficacy of simultaneous anti-TGF-β/VEGF bispecific antibody and PD-1 blockade in cancer therapy
- PMID: 37573354
- PMCID: PMC10423429
- DOI: 10.1186/s13045-023-01487-5
Synergistic efficacy of simultaneous anti-TGF-β/VEGF bispecific antibody and PD-1 blockade in cancer therapy
Abstract
Background: Recently, therapeutic antibodies against programmed cell death 1 (PD-1) and its ligand (PD-L1) have exerted potent anticancer effect in a variety of tumors. However, blocking the PD-1/PD-L1 axis alone is not sufficient to restore normal immune response. Other negative regulators of antitumor immunity, like TGF-β and VEGFA, are also involved in immune escape of tumor cells and induce immunotherapy resistance.
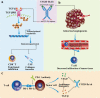
Methods: We developed a novel anti-TGF-β/VEGF bispecific antibody Y332D based on the Nano-YBODY™ technology platform. The CCK-8, flow cytometry, SBE4 luciferase reporter assay, western blotting and transwell assays were used to measure the biological activities of the anti-TGF-β moiety. The NFAT luciferase reporter assay, luminescent cell viability assay and tube formation assay were used to measure the biological activities of the anti-VEGF moiety. The in vivo anticancer efficacy of Y332D alone or in combination with PD-1 blockade was evaluated in H22, EMT-6, 4T1, and AKT/Ras-driven murine hepatocellular carcinoma tumor models. Immunofluorescent staining, flow cytometry, RNA-seq and quantitative RT-PCR were adopted to analyze the alterations in the tumor microenvironment.
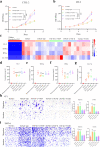
Results: Y332D could maintain specific binding affinities for TGF-β and VEGFA. Y332D almost entirely counteracted the in vitro biological functions of TGF-β and VEGFA, including immunosuppression, activated TGF-β signaling, epithelial-mesenchymal transition (EMT), activated VEGF/VEGFR signaling, HUVEC proliferation and tube formation. The in vivo experiment data demonstrated that Y332D was more effective in inhibiting tumor growth and metastasis than anti-TGF-β and anti-VEGF monotherapies. In combination therapies, Y332D plus PD-1 blockade exhibited the most potent and durable anticancer effect. Mechanistically, Y332D plus PD-1 blockade upregulated the density and function of tumor-infiltrating lymphocytes and exerted reinvigorated antitumor immunity.
Conclusion: Y332D could simultaneously block TGF-β and VEGF signalings. In comparison with the monotherapies, Y332D combined with PD-1 blockade exerts superior antitumor effect through improving immune microenvironment.
Keywords: Bispecific antibody; Cancer immunotherapy; PD-1; TGF-β; The tumor microenvironment; VEGF.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
QH, RY, LZ, JS, JZ and PZ were employees of Wuhan YZY Biopharma Co., Ltd.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

