Bojungikgi-tang improves skin barrier function and immune response in atopic dermatitis mice fed a low aryl hydrocarbon receptor ligand diet
- PMID: 37573390
- PMCID: PMC10423424
- DOI: 10.1186/s13020-023-00806-9
Bojungikgi-tang improves skin barrier function and immune response in atopic dermatitis mice fed a low aryl hydrocarbon receptor ligand diet
Abstract
Background: The aryl hydrocarbon receptor (AhR) is a transcription factor that plays a crucial role in regulating the immune system and maintaining skin barrier function. AhR signaling is pivotal in the pathogenesis of inflammatory diseases such as atopic dermatitis (AD), and the absence of AhR ligands further contributes to the progression or worsening of AD symptoms.
Methods: AD was induced with 2,4-dinitrochlorobenzene (DNCB), and Bojungikgi-tang (BJIKT) was administered orally daily for 10 weeks. Serum IgE, splenocyte IL-4, and IFN-γ levels, skin barrier genes, and AhR target gene expressions were analyzed using RNA-sequencing analysis. Spleen tissues were extracted for fluorescence-activated cell sorting (FACS) analysis to analyze the effect of BJIKT on immune responses. A correlation analysis was conducted to analyze the correlation between immune markers and skin barrier genes and AhR target genes.
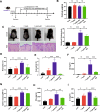
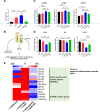
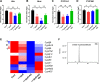
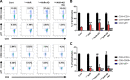
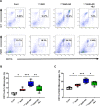
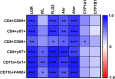
Results: BJIKT effectively improved AD symptoms in AD mice fed a low AhR ligand diet by reducing neutrophil and eosinophil counts, lowering IgE levels in the blood, and decreasing IL-4 and IFN-γ levels in the splenocytes. Additionally, BJIKT significantly reduced epithelial skin thickness and transepidermal water loss (TEWL) values and reversed the decreased expression of skin barrier genes. BJIKT also considerably altered the expression of AhR target genes, including Ahr, Ahrr, cytochrome P450 1A1 (CYP1A1), and CYP1B1. Furthermore, AhR target pathway genes were negatively correlated with immune cell subtypes, including CD4 + and CD8 + T cells and macrophages (CD11b + F4/80 +) at the systemic level.
Conclusions: BJIKT can regulate AhR activation and may help reduce inflammation in AD by regulating the expression of skin barrier genes and immune responses.
Keywords: Atopic dermatitis; BJIKT; Immune response; Inflammation; Low-AhR ligand diet; Skin barrier.
© 2023. International Society for Chinese Medicine and BioMed Central Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Fritsche E, Schäfer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

