Current incidence, severity, and management of veno-occlusive disease/sinusoidal obstruction syndrome in adult allogeneic HSCT recipients: an EBMT Transplant Complications Working Party study
- PMID: 37573397
- PMCID: PMC10622315
- DOI: 10.1038/s41409-023-02077-2
Current incidence, severity, and management of veno-occlusive disease/sinusoidal obstruction syndrome in adult allogeneic HSCT recipients: an EBMT Transplant Complications Working Party study
Abstract
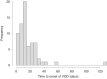
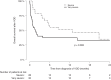
The current incidence, diagnostic policy, management, and outcome of VOD/SOS at EBMT centers were studied. All centers that had performed allogeneic HSCTs in adult patients within one defined year were invited to the study. Seventy-one centers participated with a total of 2886 allogeneic transplantations and 93 cases of VOD/SOS in 2018. The cumulative incidence of VOD/SOS at day 21 was 1.8% and at day 100 2.4%. Of 67 cases with detailed data, 52 were classical and 15 (22%) late onset (>day 21). According to the EBMT criteria, 65/67 patients had at least two VOD/SOS risk factors. The severity grades were: mild 0, moderate 3, severe 29, very severe 35. Fifty-four patients were treated with defibrotide. VOD/SOS resolved in 58% of the patients, 3/3 with moderate, 22/28 with severe, and 12/33 with very severe grade (p < 0.001). By day 100, 57% of the patients were alive; 3/3 with moderate, 22/29 with severe, and 13/35 with very severe VOD/SOS (p = 0.002). In conclusion, the incidence of VOD/SOS was low. Severe and very severe grades dominated. Very severe grade predicted poor outcome compared to severe grade further supporting the concept of early diagnosis and treatment to avoid a dismal outcome.
© 2023. The Author(s).
Conflict of interest statement
MM received research support and honoraria for lectures from Amgen, Astellas, BMS, GSK, Janssen, Jazz Pharmaceuticals, Pfizer, Takeda, Oncopeptides, Adaptive, and Sanofi. NK served as consultant and received honoraria from Jazz Pharmaceuticals. OP received research and travel support and served as a member of advisory board of Jazz Pharmaceuticals. HS reports having received personal fees from Incyte, Janssen, Novartis, Sanofi and from the Belgian Hematological Society (BHS), as well as research grants from Novartis and the BHS, both paid to her institution. She has received non-financial support from Gilead, Pfizer, the EBMT (European Society for Blood and Marrow Transplantation) and the CIBMTR (Center for International Bone Marrow Transplantation Research). She has also frequently served as a volunteer for EBMT, CIBMTR and EUPATI. RFD received honoraria as speaker for Jazz Pharmaceuticals. TR, IM, TS, JHB, AR, and GB received honoraria from Jazz Pharmaceuticals. CP, MH, EP, NS, JP, GGW, AG, SS, MA, US, AECB, JM, KH, TZ, HL-W, CK, and ZP declare no competing interests.
Figures
References
-
- Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives – a position statement from the European Society for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 2015;50:781–9. doi: 10.1038/bmt.2015.52. - DOI - PMC - PubMed
-
- Carreras E, Diaz-Beyá M, Rosiñol L, Martinez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17:1713–20. doi: 10.1016/j.bbmt.2011.06.006. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources