Computational study of the mechanical behavior of the astrocyte network and axonal compartments in the mouse optic nerve head
- PMID: 37573553
- PMCID: PMC10988382
- DOI: 10.1007/s10237-023-01752-z
Computational study of the mechanical behavior of the astrocyte network and axonal compartments in the mouse optic nerve head
Abstract
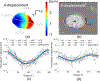
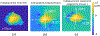
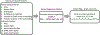
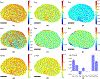
Glaucoma is a blinding disease characterized by the degeneration of the retinal ganglion cell (RGC) axons at the optic nerve head (ONH). A major risk factor for glaucoma is the intraocular pressure (IOP). However, it is currently impossible to measure the IOP-induced mechanical response of the axons of the ONH. The objective of this study was to develop a computational modeling method to estimate the IOP-induced strains and stresses in the axonal compartments in the mouse astrocytic lamina (AL) of the ONH, and to investigate the effect of the structural features on the mechanical behavior. We developed experimentally informed finite element (FE) models of six mouse ALs to investigate the effect of structure on the strain responses of the astrocyte network and axonal compartments to pressure elevation. The specimen-specific geometries of the FE models were reconstructed from confocal fluorescent images of cryosections of the mouse AL acquired in a previous study that measured the structural features of the astrocytic processes and axonal compartments. The displacement fields obtained from digital volume correlation in prior inflation tests of the mouse AL were used to determine the displacement boundary conditions of the FE models. We then applied Gaussian process regression to analyze the effects of the structural features on the strain outcomes simulated for the axonal compartments. The axonal compartments experienced, on average, 6 times higher maximum principal strain but 1800 times lower maximum principal stress compared to those experienced by the astrocyte processes. The strains experienced by the axonal compartments were most sensitive to variations in the area of the axonal compartments. Larger axonal compartments that were more vertically aligned, closer to the AL center, and with lower local actin area fraction had higher strains. Understanding the factors affecting the deformation in the axonal compartments will provide insights into mechanisms of glaucomatous axonal damage.
Keywords: Astrocytes; Axons; Gaussian process regression; Glaucoma; Optic nerve head.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Competing interests: Not application
Figures








References
-
- Bar-Kochba E, Toyjanova J, Andrews E, et al. (2015) A fast iterative digital volume correlation algorithm for large deformations. Exp Mech 55(1):261–274
-
- Bellezza A, Hart R, Burgoyne C (2000) The optic nerve head as a biomechanical structure: initial finite element modeling. Invest Ophthalmol Vis Sci 41(10):2991–3000 - PubMed
-
- Bonet J, Wood R (1997) Nonlinear continuum mechanics for finite element analysis. Cambridge university press
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

