Envonalkib versus crizotinib for treatment-naive ALK-positive non-small cell lung cancer: a randomized, multicenter, open-label, phase III trial
- PMID: 37574511
- PMCID: PMC10423717
- DOI: 10.1038/s41392-023-01538-w
Envonalkib versus crizotinib for treatment-naive ALK-positive non-small cell lung cancer: a randomized, multicenter, open-label, phase III trial
Abstract
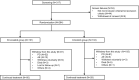
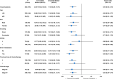
Anaplastic lymphoma kinase (ALK) rearrangements are present in about 5-6% of non-small cell lung cancer (NSCLC) cases and associated with increased risks of central nervous system (CNS) involvement. Envonalkib, a novel ALK inhibitor, demonstrated promising anti-tumor activity and safety in advanced ALK-positive NSCLC in the first-in-human phase I study. This phase III trial (ClinicalTrials.gov NCT04009317) investigated the efficacy and safety of first-line envonalkib in advanced ALK-positive NSCLC cases. Totally 264 participants were randomized 1:1 to receive envonalkib (n = 131) or crizotinib (n = 133). Median independent review committee (IRC)-assessed progression-free survival (PFS) times were 24.87 (95% confidence interval [CI]: 15.64-30.36) and 11.60 (95% CI: 8.28-13.73) months in the envonalkib and crizotinib groups, respectively (hazard ratio [HR] = 0.47, 95% CI: 0.34-0.64, p < 0.0001). IRC-assessed confirmed objective response rate (ORR) was higher (81.68% vs. 70.68%, p = 0.056) and duration of response was longer (median, 25.79 [95% CI, 16.53-29.47] vs. 11.14 [95% CI, 9.23-16.59] months, p = 0.0003) in the envonalkib group compared with the crizotinib group. In participants with baseline brain target lesions, IRC-assessed CNS-ORR was improved with envonalkib compared with crizotinib (78.95% vs. 23.81%). Overall survival (OS) data were immature, and median OS was not reached in either group (HR = 0.84, 95% CI: 0.48-1.47, p = 0.5741). The 12-month OS rates were 90.6% (95% CI, 84.0%-94.5%) and 89.4% (95% CI, 82.8%-93.6%) in the envonalkib and crizotinib groups, respectively. Grade ≥3 treatment-related adverse events were observed in 55.73% and 42.86% of participants in the envonalkib and crizotinib groups, respectively. Envonalkib significantly improved PFS and delayed brain metastasis progression in advanced ALK-positive NSCLC.
© 2023. West China Hospital, Sichuan University.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

