Changes in Bone Quality after Treatment with Etelcalcetide
- PMID: 37574661
- PMCID: PMC10637456
- DOI: 10.2215/CJN.0000000000000254
Changes in Bone Quality after Treatment with Etelcalcetide
Abstract
Introduction: Secondary hyperparathyroidism is associated with osteoporosis and fractures. Etelcalcetide is an intravenous calcimimetic for the control of hyperparathyroidism in patients on hemodialysis. Effects of etelcalcetide on the skeleton are unknown.
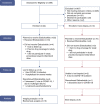
Methods: In a single-arm, open-label, 36-week prospective trial, we hypothesized that etelcalcetide improves bone quality and strength without damaging bone-tissue quality. Participants were 18 years or older, on hemodialysis ≥1 year, without calcimimetic exposure within 12 weeks of enrollment. We measured pretreatment and post-treatment areal bone mineral density by dual-energy X-ray absorptiometry, central skeleton trabecular microarchitecture by trabecular bone score, and peripheral skeleton volumetric bone density, geometry, microarchitecture, and estimated strength by high-resolution peripheral quantitative computed tomography. Bone-tissue quality was assessed using quadruple-label bone biopsy in a subset of patients. Paired t tests were used in our analysis.
Results: Twenty-two participants were enrolled; 13 completed follow-up (mean±SD age 51±14 years, 53% male, and 15% White). Five underwent bone biopsy (mean±SD age 52±16 years and 80% female). Over 36 weeks, parathyroid hormone levels declined 67%±9% ( P < 0.001); areal bone mineral density at the spine, femoral neck, and total hip increased 3%±1%, 7%±2%, and 3%±1%, respectively ( P < 0.05); spine trabecular bone score increased 10%±2% ( P < 0.001); and radius stiffness and failure load trended to a 7%±4% ( P = 0.05) and 6%±4% increase ( P = 0.06), respectively. Bone biopsy demonstrated a decreased bone formation rate (mean difference -25±4 µ m 3 / µ m 2 per year; P < 0.01).
Conclusions: Treatment with etelcalcetide for 36 weeks was associated with improvements in central skeleton areal bone mineral density and trabecular quality and lowered bone turnover without affecting bone material properties.
Clinical trial registry name and registration number: The Effect of Etelcalcetide on CKD-MBD (Parsabiv-MBD), NCT03960437.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
S. Agarwal, M.A. Aponte, and M. Bucovsky report the employment with Columbia University. M.R. Allen reports employment with Roudebush Veterans Administration; research funding from MBX Biosciences; and role on the Editorial Boards for
Figures


Comment in
-
New Insights into the Effects of Etelcalcetide on Bone Health.Clin J Am Soc Nephrol. 2023 Nov 1;18(11):1388-1390. doi: 10.2215/CJN.0000000000000316. Epub 2023 Oct 3. Clin J Am Soc Nephrol. 2023. PMID: 37791911 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

