Engineering the catalytic activity of an Antarctic PET-degrading enzyme by loop exchange
- PMID: 37574805
- PMCID: PMC10464292
- DOI: 10.1002/pro.4757
Engineering the catalytic activity of an Antarctic PET-degrading enzyme by loop exchange
Abstract
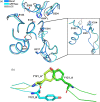
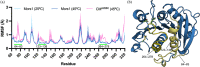
Several hydrolases have been described to degrade polyethylene terephthalate (PET) at moderate temperatures ranging from 25°C to 40°C. These mesophilic PET hydrolases (PETases) are less efficient in degrading this plastic polymer than their thermophilic homologs and have, therefore, been the subject of many protein engineering campaigns. However, enhancing their enzymatic activity through rational design or directed evolution poses a formidable challenge due to the need for exploring a large number of mutations. Additionally, evaluating the improvements in both activity and stability requires screening numerous variants, either individually or using high-throughput screening methods. Here, we utilize instead the design of chimeras as a protein engineering strategy to increase the activity and stability of Mors1, an Antarctic PETase active at 25°C. First, we obtained the crystal structure of Mors1 at 1.6 Å resolution, which we used as a scaffold for structure- and sequence-based chimeric design. Then, we designed a Mors1 chimera via loop exchange of a highly divergent active site loop from the thermophilic leaf-branch compost cutinase (LCC) into the equivalent region in Mors1. After restitution of an active site disulfide bond into this chimera, the enzyme exhibited a shift in optimal temperature for activity to 45°C and an increase in fivefold in PET hydrolysis when compared with wild-type Mors1 at 25°C. Our results serve as a proof of concept of the utility of chimeric design to further improve the activity and stability of PETases active at moderate temperatures.
Keywords: PET hydrolases; mesophilic enzymes; plastics; polyethylene terephthalate; protein engineering.
© 2023 The Protein Society.
Conflict of interest statement
The authors declare no conflict of interest exists.
Figures



References
-
- Bell EL, Smithson R, Kilbride S, Foster J, Hardy FJ, Ramachandran S, et al. Directed evolution of an efficient and thermostable PET depolymerase. Nat Catal. 2022;5:673–681.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

