Cellular immune response of SARS-CoV-2 vaccination in kidney transplant recipients: a systematic review and meta-analysis
- PMID: 37575225
- PMCID: PMC10415203
- DOI: 10.3389/fimmu.2023.1220148
Cellular immune response of SARS-CoV-2 vaccination in kidney transplant recipients: a systematic review and meta-analysis
Abstract
Background: Evidence has demonstrated inferior humoral immune responses after SARS-CoV-2 vaccination in kidney transplant recipients compared to the general population. However, data on cellular immune responses in this population have not been established.
Methods: We searched the MEDLINE, Scopus, and Cochrane databases and included studies reporting cellular immune response rates in kidney transplant recipients after receiving SARS-CoV-2 vaccines. Studies that reported factors associated with cellular immune responders or non-responders were also included (PROSPERO: CRD42022375544).
Results: From a total of 1,494 articles searched, 53 articles were included in the meta-analysis. In all, 21 studies assessed cellular immune response by interferon-γ enzyme-linked immunosorbent spot (IFN-γ ELISPOT), 22 studies used interferon-γ release assay (IGRA), and 10 studies used flow cytometric analysis. The pooled response rate after two doses (standard regimen) and three doses of vaccination was 47.5% (95%CI 38.4-56.7%) and 69.1% (95%CI 56.3-80.6%) from IFN-γ ELISPOT, 25.8% (95%CI 19.7-32.4%) and 14.7% (95%CI 8.5-22.2%) from IGRA, and 73.7% (95%CI 55.2-88.8%) and 86.5% (95%CI 75.3-94.9%) from flow cytometry, respectively. Recipients with seroconversion were associated with a higher chance of having cellular immune response (OR 2.58; 95%CI 1.89-3.54). Cellular immune response in kidney transplant recipients was lower than in dialysis patients (OR 0.24; 95%CI 0.16-0.34) and the general population (OR 0.10; 95%CI 0.07-0.14). Age and immunosuppressants containing tacrolimus or corticosteroid were associated with inferior cellular immune response.
Conclusion: Cellular immune response after SARS-CoV-2 vaccination in kidney transplant recipients was lower than in dialysis patients and the general population. Age, tacrolimus, and corticosteroid were associated with poor response. Cellular immune response should also be prioritized in vaccination studies.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022375544.
Keywords: COVID-19; Elispot; SARS-CoV-2 vaccine; T cells; cellular immune response; flow cytometry; interferon-γ; kidney transplantation.
Copyright © 2023 Udomkarnjananun, Gatechompol, Leelahavanichkul and Kerr.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



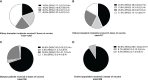
References
-
- Udomkarnjananun S, Kerr SJ, Townamchai N, Susantitaphong P, Tulvatana W, Praditpornsilpa K, et al. Mortality risk factors of COVID-19 infection in kidney transplantation recipients: a systematic review and meta-analysis of cohorts and clinical registries. Sci Rep (2021) 11(1):20073. doi: 10.1038/s41598-021-99713-y - DOI - PMC - PubMed
-
- Manothummetha K, Chuleerarux N, Sanguankeo A, Kates OS, Hirankarn N, Thongkam A, et al. Immunogenicity and risk factors associated with poor humoral immune response of SARS-CoV-2 vaccines in recipients of solid organ transplant: A systematic review and meta-analysis. JAMA Netw Open (2022) 5(4):e226822. doi: 10.1001/jamanetworkopen.2022.6822 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

