Clinical and immunological spectra of human cutaneous leishmaniasis in North Africa and French Guiana
- PMID: 37575260
- PMCID: PMC10421664
- DOI: 10.3389/fimmu.2023.1134020
Clinical and immunological spectra of human cutaneous leishmaniasis in North Africa and French Guiana
Abstract
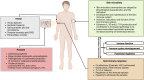
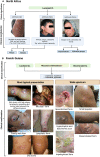
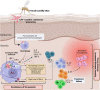
Cutaneous leishmaniasis (CL) caused by infection with the parasite Leishmania exhibits a large spectrum of clinical manifestations ranging from single healing to severe chronic lesions with the manifestation of resistance or not to treatment. Depending on the specie and multiple environmental parameters, the evolution of lesions is determined by a complex interaction between parasite factors and the early immune responses triggered, including innate and adaptive mechanisms. Moreover, lesion resolution requires parasite control as well as modulation of the pathologic local inflammation responses and the initiation of wound healing responses. Here, we have summarized recent advances in understanding the in situ immune response to cutaneous leishmaniasis: i) in North Africa caused by Leishmania (L.) major, L. tropica, and L. infantum, which caused in most cases localized autoresolutives forms, and ii) in French Guiana resulting from L. guyanensis and L. braziliensis, two of the most prevalent strains that may induce potentially mucosal forms of the disease. This review will allow a better understanding of local immune parameters, including cellular and cytokines release in the lesion, that controls infection and/or protect against the pathogenesis in new world compared to old world CL.
Keywords: L. braziliensis; L. guyanensis; L. infantum; L. major; L. tropica; clinical manifestation; cutaneous leishmaniasis (CL); local immune response.
Copyright © 2023 Saidi, Blaizot, Prévot, Aoun, Demar, Cazenave, Bouratbine and Pied.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Clinical and parasitological features of Leishmania infection among gold miners in the Oiapoque basin, an international Brazil-French Guiana border.PLoS Negl Trop Dis. 2024 Jun 26;18(6):e0012210. doi: 10.1371/journal.pntd.0012210. eCollection 2024 Jun. PLoS Negl Trop Dis. 2024. PMID: 38923969 Free PMC article.
-
Use of the intramuscular route to administer pentamidine isethionate in Leishmania guyanensis cutaneous leishmaniasis increases the risk of treatment failure.Travel Med Infect Dis. 2018 Jul-Aug;24:31-36. doi: 10.1016/j.tmaid.2018.02.010. Epub 2018 Mar 1. Travel Med Infect Dis. 2018. PMID: 29482012
-
[Diversity of Leishmania Strains Isolated from Cutaneous Leishmaniasis Patients in Turkey and its Reflection to Clinics in Mice Model].Mikrobiyol Bul. 2020 Jul;54(3):429-443. doi: 10.5578/mb.69391. Mikrobiyol Bul. 2020. PMID: 32755519 Turkish.
-
[Leishmania (Viannia) braziliensis Vianna, 1911 in French Guiana. Clinical, therapeutic and epidemiological considerations in the ninth human diagnosed case].Bull Soc Pathol Exot. 1996;89(5):341-4. Bull Soc Pathol Exot. 1996. PMID: 9264733 Review. French.
-
[CUTANEOUS LEISHMANIASIS IN ISRAEL 2016 - AN UPDATE].Harefuah. 2016 Oct;155(10):626-631. Harefuah. 2016. PMID: 28530056 Review. Hebrew.
Cited by
-
Biomarker Insights: Evaluation of Presepsin, Apelin, and Irisin Levels in Cutaneous Leishmaniasis.Diagnostics (Basel). 2024 Dec 20;14(24):2869. doi: 10.3390/diagnostics14242869. Diagnostics (Basel). 2024. PMID: 39767230 Free PMC article.
-
Cutaneous Leishmaniasis Hampers COVID-19: A Controlled Cross-Sectional Study in High-Burden Endemic Areas of Iran.J Epidemiol Glob Health. 2024 Mar;14(1):142-153. doi: 10.1007/s44197-023-00179-0. Epub 2024 Jan 8. J Epidemiol Glob Health. 2024. PMID: 38190050 Free PMC article.
-
In Situ versus Systemic Immune Response in the Pathogenesis of Cutaneous Leishmaniasis.Pathogens. 2024 Feb 23;13(3):199. doi: 10.3390/pathogens13030199. Pathogens. 2024. PMID: 38535542 Free PMC article.
-
Overview of Research on Leishmaniasis in Africa: Current Status, Diagnosis, Therapeutics, and Recent Advances Using By-Products of the Sargassaceae Family.Pharmaceuticals (Basel). 2024 Apr 18;17(4):523. doi: 10.3390/ph17040523. Pharmaceuticals (Basel). 2024. PMID: 38675483 Free PMC article.
-
A Quantitative Polymerase Chain Reaction (qPCR) Protocol Predictive of Treatment Outcome in Cutaneous Leishmaniasis Caused by Leishmania braziliensis.Open Forum Infect Dis. 2025 Jan 15;12(2):ofae749. doi: 10.1093/ofid/ofae749. eCollection 2025 Feb. Open Forum Infect Dis. 2025. PMID: 39981068 Free PMC article.
References
-
- Ruiz-Postigo JA, Jain S, Maia-Elkhoury AMAN, Valadas S, Warusavithana S, Osman M, et al. . Global leishmaniasis surveillance: 2019-2020, a baseline for the 2030 Roadmap/Surveillance mondiale de la leishmaniose: 2019-2020, une periode de reference pour la feuille de route a l’horizon 2030. Weekly Epidemiological Rec (2021) 96:401–19.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

