Lipedema stage affects adipocyte hypertrophy, subcutaneous adipose tissue inflammation and interstitial fibrosis
- PMID: 37575263
- PMCID: PMC10417720
- DOI: 10.3389/fimmu.2023.1223264
Lipedema stage affects adipocyte hypertrophy, subcutaneous adipose tissue inflammation and interstitial fibrosis
Abstract
Introduction: Lipedema is a painful subcutaneous adipose tissue (SAT) disease characterized by adipocyte hypertrophy, immune cell recruitment, and fibrosis in the affected areas. These features are thought to contribute to the development and progression of the condition. However, the relationship between lipedema disease stage and the associated adipose tissue changes has not been determined so far.
Methods: SAT biopsies of 32 lipedema patients, ranging across the pathological stages I to III, and 14 BMI- and age-matched controls were harvested from lipedema-affected thighs and non-symptomatic lower abdominal regions. Histological and immunohistochemical (IHC) staining and expression analysis of markers for adipogenesis, immunomodulation, and fibrosis were performed on the tissue biopsies.
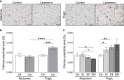
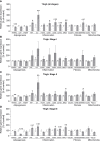
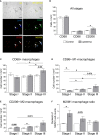
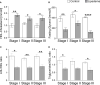
Results: Lipedema patients showed increased adipocyte areas and a stage-dependent shift towards larger cell sizes in the thighs. Lipedema SAT was linked with increased interstitial collagen accumulation in the thighs, but not the lower abdominal region when compared to controls. There was a trend toward progressive SAT fibrosis of the affected thighs with increasing lipedema stage. Elevated gene expression levels of macrophage markers were found for thigh SAT biopsies, but not in the abdominal region. IHC staining of lipedema thigh biopsies confirmed a transiently elevated macrophage polarization towards an M2-like (anti-inflammatory) phenotype.
Conclusions: In summary, lipedema SAT is associated with stage-dependent adipocyte hypertrophy, stage-progressive interstitial fibrosis and elevated proportion of M2-like macrophages. The character of the inflammatory response differs from primary obesity and may possess an essential role in the development of lipedema.
Keywords: adipose tissue; fibrosis; inflammation; lipedema; lipoedema; macrophages.
Copyright © 2023 Kruppa, Gohlke, Łapiński, Garcia-Carrizo, Soultoukis, Infanger, Schulz and Ghods.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Szolnoky G, Nagy N, Kovacs RK, Dosa-Racz E, Szabo A, Barsony K, et al. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology (2008) 41(4):161–6. - PubMed
-
- Szolnoky G, Borsos B, Barsony K, Balogh M, Kemeny L. Complete decongestive physiotherapy with and without pneumatic compression for treatment of lipedema: a pilot study. Lymphology (2008) 41(1):40–4. - PubMed
-
- Szolnoky G, Varga E, Varga M, Tuczai M, Dosa-Racz E, Kemeny L. Lymphedema treatment decreases pain intensity in lipedema. Lymphology (2011) 44(4):178–82. - PubMed
-
- Kruppa P, Georgiou I, Schmidt J, Infanger M, Ghods M. A 10-Year Retrospective before-and-after Study of Lipedema Surgery: Patient-Reported Lipedema-Associated Symptom Improvement after Multistage Liposuction. Plast reconstructive surgery. (2022) 149(3):529e–41e. doi: 10.1097/PRS.0000000000008880 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

