Phase II Window Study of Olaparib Alone or with Cisplatin or Durvalumab in Operable Head and Neck Cancer
- PMID: 37575280
- PMCID: PMC10414130
- DOI: 10.1158/2767-9764.CRC-23-0051
Phase II Window Study of Olaparib Alone or with Cisplatin or Durvalumab in Operable Head and Neck Cancer
Abstract
Purpose: We conducted a phase II randomized noncomparative window of opportunity (WOO) trial to evaluate the inhibition of cellular proliferation and the modulation of immune microenvironment after treatment with olaparib alone or in combination with cisplatin or durvalumab in patients with operable head and neck squamous cell carcinoma (HNSCC).
Experimental design: Forty-one patients with HNSCC were randomized to cisplatin plus olaparib (arm A), olaparib alone (arm B), no treatment (arm C) or durvalumab plus olaparib (arm D). The primary endpoint was to evaluate the percentage of patients in each arm that achieved a reduction of at least 25% in Ki67. Secondary endpoints included objective response rate (ORR), safety, and pathologic complete response (pCR) rate. Paired baseline and resection tumor biopsies and blood samples were evaluated for prespecified biomarkers.
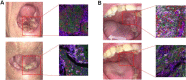
Results: A decrease in Ki67 of at least 25% was observed in 44.8% of treated patients, as measured by quantitative immunofluorescence. The ORR among treated patients was 12.1%. pCR was observed in 2 patients. Two serious adverse events occurred in 2 patients.Programmed death ligand 1 (PD-L1) levels [combined positive score (CPS)] were significantly higher after treatment in arms A and D. Expression of CD163 and colony-stimulating factor 1 receptor (CSF1R) genes, markers of M2 macrophages, increased significantly posttreatment whereas the expression of CD80, a marker of M1 macrophages, decreased.
Conclusion: Preoperative olaparib with cisplatin or alone or with durvalumab was safe in the preoperative setting and led to decrease in Ki67 of at least 25% in 44.8% of treated patients. Olaparib-based treatment modulates the tumor microenvironment leading to upregulation of PD-L1 and induction of protumor features of macrophages.
Significance: HNSCC is characterized by defective DNA repair pathways and immunosuppressive tumor microenvironment. PARP inhibitors, which promote DNA damage and "reset" the inflammatory tumor microenvironment, can establish an effective antitumor response. This phase II WOO trial in HNSCC demonstrated the immunomodulatory effects of PARP inhibitor-induced DNA damage. In this chemo-naïve population, PARP inhibitor-based treatment, reduced tumor cell proliferation and modulated tumor microenvironment. After olaparib upregulation of PD-L1 and macrophages, suggests that combinatorial treatment might be beneficial.
Synopsis: Our WOO study demonstrates that preoperative olaparib results in a reduction in Ki67, upregulation of PD-L1 CPS, and induction of protumor features of macrophages in HNSCC.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures


References
-
- Pignon JP, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. - PubMed
-
- Sliwinski T, Markiewicz L, Rusin P, Dziki L, Milonski J, Olszewski J, et al. Impaired nucleotide excision repair pathway as a possible factor in pathogenesis of head and neck cancer. Mutat Res 2011;716:51–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

