Functional Analysis of Plant Monosaccharide Transporters Using a Simple Growth Complementation Assay in Yeast
- PMID: 37575400
- PMCID: PMC10415198
- DOI: 10.21769/BioProtoc.4733
Functional Analysis of Plant Monosaccharide Transporters Using a Simple Growth Complementation Assay in Yeast
Abstract
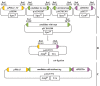
The study of genes and their products is an essential prerequisite for fundamental research. Characterization can be achieved by analyzing mutants or overexpression lines or by studying the localization and substrate specificities of the resulting proteins. However, functional analysis of specific proteins in complex eukaryotic organisms can be challenging. To overcome this, the use of heterologous systems to express genes and analyze the resulting proteins can save time and effort. Yeast is a preferred heterologous model organism: it is easy to transform, and tools for genomics, engineering, and metabolomics are already available. Here, we describe a well-established and simple method to analyze the activity of plant monosaccharide transporters in the baker's yeast, Saccharomyces cerevisiae, using a simple growth complementation assay. We used the famous hexose-transport-deficient yeast strain EBY.VW4000 to express candidate plant monosaccharide transporters and analyzed their transport activity. This assay does not require any radioactive labeling of substrates and can be easily extended for quantitative analysis using growth curves or by analyzing the transport rates of fluorescent substrates like the glucose analog 2-NBDG. Finally, to further simplify the cloning of potential candidate transporters, we provide level 0 modular cloning (MoClo) modules for efficient and simple Golden Gate cloning. This approach provides a convenient tool for the functional analysis of plant monosaccharide transporters in yeast. Key features Comprehensive, simple protocol for analysis of plant monosaccharide transporters in yeast Includes optional MoClo parts for cloning with Golden Gate method Includes protocol for the production and transformation of competent yeast cells Does not require hazardous solutions, radiolabeled substrates, or specialized equipment.
Keywords: Drop-out assay; EBY.VW4000; Heterologous expression; Plant monosaccharide transporters; Yeast.
©Copyright : © 2023 The Authors; This is an open access article under the CC BY-NC license.
Conflict of interest statement
Competing interestsThe authors declare to have no competing interests.
Figures


Similar articles
-
A Growth-Based Screening System for Hexose Transporters in Yeast.Methods Mol Biol. 2018;1713:123-135. doi: 10.1007/978-1-4939-7507-5_10. Methods Mol Biol. 2018. PMID: 29218522
-
The genome sequence of the popular hexose-transport-deficient Saccharomyces cerevisiae strain EBY.VW4000 reveals LoxP/Cre-induced translocations and gene loss.FEMS Yeast Res. 2015 Mar;15(2):fou004. doi: 10.1093/femsyr/fou004. FEMS Yeast Res. 2015. PMID: 25673752
-
Characterization of hexose transporters in Yarrowia lipolytica reveals new groups of Sugar Porters involved in yeast growth.Fungal Genet Biol. 2017 Mar;100:1-12. doi: 10.1016/j.fgb.2017.01.001. Epub 2017 Jan 5. Fungal Genet Biol. 2017. PMID: 28064038
-
The molecular genetics of hexose transport in yeasts.FEMS Microbiol Rev. 1997 Aug;21(1):85-111. doi: 10.1111/j.1574-6976.1997.tb00346.x. FEMS Microbiol Rev. 1997. PMID: 9299703 Review.
-
Monosaccharide transporters in plants: structure, function and physiology.Biochim Biophys Acta. 2000 May 1;1465(1-2):263-74. doi: 10.1016/s0005-2736(00)00143-7. Biochim Biophys Acta. 2000. PMID: 10748259 Review.
References
-
- Bezrutczyk M., Yang J., Eom J. S., Prior M., David. Sosso, Hartwig T., Boris. Szurek, Oliva R., Vera-Cruz C., White F. F., et al. .(2018). Sugar flux and signaling in plant-microbe interactions. Plant J 93(4): 675-685. - PubMed
-
- Boles E.(2003). Yeast as a Model System for Studying Glucose Transport. In: Sibley, D. R. and Quick, M. W.(Eds.). Transmembrane Transporters. Wiley‐Liss, Inc.
LinkOut - more resources
Full Text Sources
Research Materials

