APOE4, Age, and Sex Regulate Respiratory Plasticity Elicited by Acute Intermittent Hypercapnic-Hypoxia
- PMID: 37575478
- PMCID: PMC10413930
- DOI: 10.1093/function/zqad026
APOE4, Age, and Sex Regulate Respiratory Plasticity Elicited by Acute Intermittent Hypercapnic-Hypoxia
Abstract
Rationale: Acute intermittent hypoxia (AIH) shows promise for enhancing motor recovery in chronic spinal cord injuries and neurodegenerative diseases. However, human trials of AIH have reported significant variability in individual responses.
Objectives: Identify individual factors (eg, genetics, age, and sex) that determine response magnitude of healthy adults to an optimized AIH protocol, acute intermittent hypercapnic-hypoxia (AIHH).
Methods: In 17 healthy individuals (age = 27 ± 5 yr), associations between individual factors and changes in the magnitude of AIHH (15, 1-min O2 = 9.5%, CO2 = 5% episodes) induced changes in diaphragm motor-evoked potential (MEP) amplitude and inspiratory mouth occlusion pressures (P0.1) were evaluated. Single nucleotide polymorphisms (SNPs) in genes linked with mechanisms of AIH induced phrenic motor plasticity (BDNF, HTR2A, TPH2, MAOA, NTRK2) and neuronal plasticity (apolipoprotein E, APOE) were tested. Variations in AIHH induced plasticity with age and sex were also analyzed. Additional experiments in humanized (h)ApoE knock-in rats were performed to test causality.
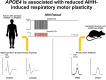
Results: AIHH-induced changes in diaphragm MEP amplitudes were lower in individuals heterozygous for APOE4 (i.e., APOE3/4) compared to individuals with other APOE genotypes (P = 0.048) and the other tested SNPs. Males exhibited a greater diaphragm MEP enhancement versus females, regardless of age (P = 0.004). Additionally, age was inversely related with change in P0.1 (P = 0.007). In hApoE4 knock-in rats, AIHH-induced phrenic motor plasticity was significantly lower than hApoE3 controls (P < 0.05).
Conclusions: APOE4 genotype, sex, and age are important biological determinants of AIHH-induced respiratory motor plasticity in healthy adults.
Addition to knowledge base: AIH is a novel rehabilitation strategy to induce functional recovery of respiratory and non-respiratory motor systems in people with chronic spinal cord injury and/or neurodegenerative disease. Figure 5 Since most AIH trials report considerable inter-individual variability in AIH outcomes, we investigated factors that potentially undermine the response to an optimized AIH protocol, AIHH, in healthy humans. We demonstrate that genetics (particularly the lipid transporter, APOE), age and sex are important biological determinants of AIHH-induced respiratory motor plasticity.
Keywords: APOE4; age; biomarkers; genetics; intermittent hypercapnic-hypoxia; respiratory neuroplasticity; sex.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
None declared.
Figures


 = participant (S6) was identified as the most influential point (Cook’s D > 4) in the percentage change in diaphragm MEP amplitudes, therefore, the data were not included in group analyses.
= participant (S6) was identified as the most influential point (Cook’s D > 4) in the percentage change in diaphragm MEP amplitudes, therefore, the data were not included in group analyses.
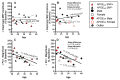
 = participant (S6) was identified as the most influential point (Cook’s D > 4) in the percentage change in diaphragm MEP amplitudes, therefore, the data was not included in group analyses.
= participant (S6) was identified as the most influential point (Cook’s D > 4) in the percentage change in diaphragm MEP amplitudes, therefore, the data was not included in group analyses.Update of
-
APOE4, Age & Sex Regulate Respiratory Plasticity Elicited By Acute Intermittent Hypercapnic-Hypoxia.bioRxiv [Preprint]. 2023 Jan 7:2023.01.06.522840. doi: 10.1101/2023.01.06.522840. bioRxiv. 2023. Update in: Function (Oxf). 2023 Jun 13;4(5):zqad026. doi: 10.1093/function/zqad026. PMID: 36711653 Free PMC article. Updated. Preprint.
Comment in
-
Intermittent Hypoxia and Respiratory Plasticity: The Good, the Bad, and the Unknown.Function (Oxf). 2023 Sep 4;4(6):zqad045. doi: 10.1093/function/zqad045. eCollection 2023. Function (Oxf). 2023. PMID: 37753181 Free PMC article. No abstract available.
References
-
- Vermeulen TD, et al. Acute intermittent hypercapnic hypoxia and cerebral neurovascular coupling in males and females. Exp Neurol. 2020;334:113441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

