Micromolded honeycomb scaffold design to support the generation of a bilayered RPE and photoreceptor cell construct
- PMID: 37575875
- PMCID: PMC10415596
- DOI: 10.1016/j.bioactmat.2023.07.019
Micromolded honeycomb scaffold design to support the generation of a bilayered RPE and photoreceptor cell construct
Abstract
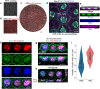
Age-related macular degeneration (AMD) causes blindness due to loss of retinal pigment epithelium (RPE) and photoreceptors (PRs), which comprise the two outermost layers of the retina. Given the small size of the macula and the importance of direct contact between RPE and PRs, the use of scaffolds for targeted reconstruction of the outer retina in later stage AMD and other macular dystrophies is particularly attractive. We developed microfabricated, honeycomb-patterned, biodegradable poly(glycerol sebacate) (PGS) scaffolds to deliver organized, adjacent layers of RPE and PRs to the subretinal space. Furthermore, an optimized process was developed to photocure PGS, shortening scaffold production time from days to minutes. The resulting scaffolds robustly supported the seeding of human pluripotent stem cell-derived RPE and PRs, either separately or as a dual cell-layered construct. These advanced, economical, and versatile scaffolds can accelerate retinal cell transplantation efforts and benefit patients with AMD and other retinal degenerative diseases.
Keywords: Microfabrication; Retina; Scaffolds; Stem cells; Tissue engineering.
© 2023 The Authors.
Conflict of interest statement
D.M.G. is an inventor on patents related to this work filed by the Wisconsin Alumni Research Foundation, Madison, WI (no. 9,752,119, filed 29 April 2016, published 5 September 2017) (no. 9,328,328, filed 24 August 2010, published 3 May 2016). D.M.G., Z.M., M.J.P., and S.G. are inventors on a patent filed by the Wisconsin Alumni Research Foundation, Madison, WI (no. 2017/0226459, filed 5 February 2016, published 10 August 2017). D.M.G., Z.M., M.J.P., S.G., and I.-K.L. are inventors on patents filed by the Wisconsin Alumni Research Foundation, Madison, WI (no. 2020/0010799, filed 9 July 2019, published 01 September 2020) (serial no. 17/769549, filed 10 October 2020, pending). D.M.G. and M.J.P. have an ownership interest in Opsis Therapeutics LLC, which has licensed the technology to generate 3D retinal organoids from pluripotent stem cell sources reported in this publication. The authors declare that they have no other competing interests.
Figures






References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85(3):845–881. - PubMed
-
- Rein D.B., Wittenborn J.S., Zhang X., Honeycutt A.A., Lesesne S.B., Saaddine J. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch. Ophthalmol. 2009;127(4):533–540. - PubMed
LinkOut - more resources
Full Text Sources

