Development and characterization of transfontanelle photoacoustic imaging system for detection of intracranial hemorrhages and measurement of brain oxygenation: Ex-vivo
- PMID: 37575972
- PMCID: PMC10413353
- DOI: 10.1016/j.pacs.2023.100538
Development and characterization of transfontanelle photoacoustic imaging system for detection of intracranial hemorrhages and measurement of brain oxygenation: Ex-vivo
Abstract
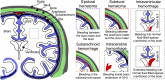
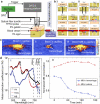
We have developed and optimized an imaging system to study and improve the detection of brain hemorrhage and to quantify oxygenation. Since this system is intended to be used for brain imaging in neonates through the skull opening, i.e., fontanelle, we called it, Transfontanelle Photoacoustic Imaging (TFPAI) system. The system is optimized in terms of optical and acoustic designs, thermal safety, and mechanical stability. The lower limit of quantification of TFPAI to detect the location of hemorrhage and its size is evaluated using in-vitro and ex-vivo experiments. The capability of TFPAI in measuring the tissue oxygenation and detection of vasogenic edema due to brain blood barrier disruption are demonstrated. The results obtained from our experimental evaluations strongly suggest the potential utility of TFPAI, as a portable imaging modality in the neonatal intensive care unit. Confirmation of these findings in-vivo could facilitate the translation of this promising technology to the clinic.
Keywords: Hemorrhage; Neonates; Oxygenation; Photoacoustic imaging; Transfontanelle.
© 2023 Published by Elsevier GmbH.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kamran Avanaki reports financial support was provided by National Institutes of Health. Kamran Avanaki has patent issued to Wayne State University. Juri G. Gelovani has patent issued to Wayne State University.
Figures







References
-
- Heron Melonie, Sutton, Paul D., Xu Jiaquan, Ventura Stephanie J., Strobino Donna M., Guyer Bernard. Annual summary of vital statistics: 2007. Pediatrics. 2010;125(1):4–15. - PubMed
-
- Griffiths, Ruth, The abilities of babies: a study in mental measurement. 1954.
-
- Luiz D.M., Foxcroft C.D., Stewart R. The construct validity of the Griffiths Scales of Mental Development. Child.: Care Health Dev. 2001;27(1):73–83. - PubMed
-
- M Levene. In: Roberton's Textbook of Neonatology. fourth ed. JM R., editor. Elsevier; Philadelphia: 2005. Intracranial haemorrhage at term.
Grants and funding
LinkOut - more resources
Full Text Sources

