Extracellular vesicles: cross-organismal RNA trafficking in plants, microbes, and mammalian cells
- PMID: 37575974
- PMCID: PMC10419970
- DOI: 10.20517/evcna.2023.10
Extracellular vesicles: cross-organismal RNA trafficking in plants, microbes, and mammalian cells
Abstract
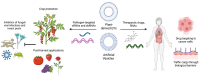
Extracellular vesicles (EVs) are membrane-enclosed nanometer-scale particles that transport biological materials such as RNAs, proteins, and metabolites. EVs have been discovered in nearly all kingdoms of life as a form of cellular communication across different cells and between interacting organisms. EV research has primarily focused on EV-mediated intra-organismal transport in mammals, which has led to the characterization of a plethora of EV contents from diverse cell types with distinct and impactful physiological effects. In contrast, research into EV-mediated transport in plants has focused on inter-organismal interactions between plants and interacting microbes. However, the overall molecular content and functions of plant and microbial EVs remain largely unknown. Recent studies into the plant-pathogen interface have demonstrated that plants produce and secrete EVs that transport small RNAs into pathogen cells to silence virulence-related genes. Plant-interacting microbes such as bacteria and fungi also secrete EVs which transport proteins, metabolites, and potentially RNAs into plant cells to enhance their virulence. This review will focus on recent advances in EV-mediated communications in plant-pathogen interactions compared to the current state of knowledge of mammalian EV capabilities and highlight the role of EVs in cross-kingdom RNA interference.
Keywords: Extracellular vesicles; cross-kingdom RNA interference; plant-microbial interaction; small RNAs; spray-induced gene silencing.
Conflict of interest statement
Conflicts of interest All authors declared that there are no conflicts of interest.
Figures


References
-
- Jensen WA. The composition and ultrastructure of the nucellus in cotton. J Ultrastruct Res. 1965;13:112–28. doi: 10.1016/S0022-5320(65)80092-2. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
