Evaluation of national institute for health and care excellence guidance for ruptured abdominal aortic aneurysms by emulating a hypothetical target trial
- PMID: 37576114
- PMCID: PMC10419256
- DOI: 10.3389/fcvm.2023.1219744
Evaluation of national institute for health and care excellence guidance for ruptured abdominal aortic aneurysms by emulating a hypothetical target trial
Abstract
Objective: This retrospective study evaluates the performance of UK National Institute for Health and Care Excellence (NICE) Guidelines on management of ruptured abdominal aortic aneurysms in a "real world setting" by emulating a hypothetical target trial with data from two European Aortic Centers.
Methods: Clinical data was retrospectively collected for all patients who had undergone ruptured endovascular aneurysm repair (rEVAR) and ruptured open surgical repair (rOSR). Survival analysis was performed comparing NICE compliance to usual care strategy. NICE compliers were defined as: female patients undergoing rEVAR; male patients >70 years old undergoing rEVAR; and male patients ≤70 years old undergoing rOSR. Hemodynamic instability was considered additionally.
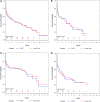
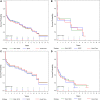
Results: This multicenter study included 298 patients treated for rAAA. The majority of patients were treated with rOSR (186 rOSR vs. 112 rEVAR). Overall, 184 deaths (68 [37%] with rEVAR and 116 [63%] with rOSR) were observed during the study period. Overall survival under usual care was 69.2% at 30 days, 56.5% at one year, and 42.4% at 5 years. NICE compliance gave survival outcomes of 73.1% at 30 days, 60.2% at 1 year and 42.9% at 5 years. The risk ratios at these time points, comparing NICE-compliance to usual care, were 0.88, 0.92 and 0.99, respectively.
Conclusions: We support NICE recommendations to manage men below the age of 71 years and hemodynamic stability with rOSR. There was a slight survival advantage for NICE compliers overall, in men >70 years and women of all ages.
Keywords: aortic aneurysm; endovascular aneurysm repair; guidelines; infrarenal; open repair; ruptured.
© 2023 Eilenberg, Waduud, Davies, Bailey, Scott, Wolf, Sotir, Lakowitsch, Kaider, Heinze, Brostjan, Domenig and Neumayer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Hinchliffe RJ, Bruijstens L, MacSweeney ST, Braithwaite BD. A randomised trial of endovascular and open surgery for ruptured abdominal aortic aneurysm—results of a pilot study and lessons learned for future studies. Eur J Vasc Endovasc Surg. (2006) 32(5):506–13. discussion 14–5. 10.1016/j.ejvs.2006.05.016 - DOI - PubMed
LinkOut - more resources
Full Text Sources

