Combination of human platelet lysate and 3D gelatin scaffolds to enhance osteogenic differentiation of human amniotic fluid derived mesenchymal stem cells
- PMID: 37576189
- PMCID: PMC10413082
- DOI: 10.1016/j.heliyon.2023.e18599
Combination of human platelet lysate and 3D gelatin scaffolds to enhance osteogenic differentiation of human amniotic fluid derived mesenchymal stem cells
Abstract
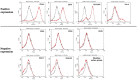
Bone disorders are major health issues requiring specialized care; however, the traditional bone grafting method had several limitations. Thus, bone tissue engineering has become a potential alternative. In therapeutic treatments, using fetal bovine serum (FBS) as a culture supplement may result in the risk of contamination and host immunological response; therefore, human platelet lysate (hPL) has been considered a viable alternative source. This study attempted to compare the effectiveness and safety of different culture supplements, either FBS or hPL, on the osteoblastic differentiation potential of mesenchymal stem cells derived from human amniotic fluid (hAF-MSCs) under a three-dimensional gelatin scaffold. The results indicate that hAF-MSCs have the potential to be used in clinical applications as they meet the criteria for mesenchymal stem cells based on their morphology, the expression of a particular surface antigen, their proliferation ability, and their capacity for multipotent differentiation. After evaluation by MTT and Alamar blue proliferation assay, 10% of hPL was selected. The osteogenic differentiation of hAF-MSCs under three-dimensional gelatin scaffold using osteogenic-induced media supplemented with hPL was achievable and markedly stimulated osteoblast differentiation. Moreover, the expressions of osteoblastogenic related genes, including OCN, ALP, and COL1A1, exhibited the highest degree of expression under hPL-supplemented circumstances when compared with the control and the FBS-supplemented group. The induced cells under hPL-supplemented conditions also presented the highest ALP activity level and the greatest degree of calcium accumulation. These outcomes would indicate that hPL is a suitable substitute for animal derived serum. Importantly, osteogenic differentiation of human amniotic fluid derived mesenchymal stem cells using hPL-supplemented media and three-dimensional scaffolds may open the door to developing an alternative construct for repairing bone defects.
Keywords: Human amniotic fluid mesenchymal stem cells; Human platelet lysate; Osteoblast-like cells; Osteogenic differentiation; Scaffold; Tissue engineering.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

