Hypertrophy of the right ventricle by pulmonary artery banding in rats: a study of structural, functional, and transcriptomics alterations in the right and left ventricles
- PMID: 37576341
- PMCID: PMC10414540
- DOI: 10.3389/fphys.2023.1129333
Hypertrophy of the right ventricle by pulmonary artery banding in rats: a study of structural, functional, and transcriptomics alterations in the right and left ventricles
Abstract
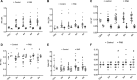
Introduction: Right ventricular remodeling with subsequent functional impairment can occur in some clinical conditions in adults and children. The triggering factors, molecular mechanisms, and, especially, the evolution over time are still not well known. Left ventricular (LV) changes associated with right ventricular (RV) remodeling are also poorly understood. Objectives: The study aimed to evaluate RV morphological, functional, and gene expression parameters in rats submitted to pulmonary artery banding compared to control rats, with the temporal evolution of these parameters, and to analyze the influence of RV remodeling by pulmonary artery banding in rats and their controls over time on LV geometry, histology, gene expression, and functional performance. Methods: Healthy 6-week-old male Wistar-EPM rats weighing 170-200 g were included. One day after the echocardiogram, depending on the animals undergoing the pulmonary artery banding (PAB) procedure or not (control group), they were then randomly divided into subgroups according to the follow-up time: 72 h, or 2, 4, 6, or 8 weeks. In each subgroup, the following were conducted: a new echocardiogram, a hemodynamic study, the collection of material for morphological analysis (hypertrophy and fibrosis), and molecular biology (gene expression). The results were presented as the mean ± standard deviation of the mean. A two-way ANOVA and Tukey post-test compared the variables of the subgroups and evolution follow-up times. The adopted significance level was 5%. Results: There was no significant difference among the subgroups in the percentage of water in both the lungs and the liver (the percentage of water in the lungs ranged from 76% to 78% and that of the liver ranged from 67% to 71%). The weight of the right chambers was significantly higher in PAB animals in all subgroups (RV PAB weighed from 0.34 to 0.48 g, and control subjects, from 0.17 to 0.20 g; right atrium (RA) with PAB from 0.09 to 0.14 g; and control subjects from 0.02 to 0.03 g). In the RV of PAB animals, there was a significant increase in myocyte nuclear volume (97 μm3-183.6 μm3) compared to control subjects (34.2 μm3-57.2 μm3), which was more intense in subgroups with shorter PAB follow-up time, and the fibrosis percentage (5.9%-10.4% vs. 0.96%-1.18%) was higher as the PAB follow-up time was longer. In the echocardiography result, there was a significant increase in myocardial thickness in all PAB groups (0.09-0.11 cm compared to control subjects-0.04-0.05 cm), but there was no variation in RV diastolic diameter. From 2 to 8 weeks of PAB, the S-wave (S') (0.031 cm/s and 0.040 cm/s), and fractional area change (FAC) (51%-56%), RV systolic function parameters were significantly lower than those of the respective control subjects (0.040 cm/s to 0.050 cm/s and 61%-67%). Furthermore, higher expression of genes related to hypertrophy and extracellular matrix in the initial subgroups and apoptosis genes in the longer follow-up PAB subgroups were observed in RV. On the other hand, LV weight was not different between animals with and without PAB. The nuclear volume of the PAB animals was greater than that of the control subjects (74 μm3-136 μm3; 40.8 μm3-46.9 μm3), and the percentage of fibrosis was significantly higher in the 4- and 8-week PAB groups (1.2% and 2.2%) compared to the control subjects (0.4% and 0.7%). Echocardiography showed that the diastolic diameter and LV myocardial thickness were not different between PAB animals and control subjects. Measurements of isovolumetric relaxation time and E-wave deceleration time at the echocardiography were different between PAB animals and control subjects in all subgroups, but there were no changes in diastolic function in the hemodynamic study. There was also increased expression of genes related to various functions, particularly hypertrophy. Conclusion: 1) Rats submitted to pulmonary artery banding presented RV remodeling compatible with hypertrophy. Such alterations were mediated by increased gene expression and functional alterations, which coincide with the onset of fibrosis. 2) Structural changes of the RV, such as weight, myocardial thickness, myocyte nuclear volume, and degree of fibrosis, were modified according to the time of exposure to pulmonary artery banding and related to variations in gene expression, highlighting the change from an alpha to a beta pattern from early to late follow-up times. 3) The study suggests that the left ventricle developed histological alterations accompanied by gene expression modifications simultaneously with the alterations found in the right ventricle.
Keywords: hypertrophy; pulmonary artery banding; rats; remodeling; right ventricle.
Copyright © 2023 Silva, Antonio, Dos Santos, Serra, Feliciano, Junior, Ihara, Tucci and Moises.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Amanuma S., Sekiguchi M., Ogasawara S., Honda M., Hosoda S. (1994). Biventricular endomyocardial biopsy findings in essential hypertension of graded severity. Postgrad. Med. 70, S67–S71. - PubMed
-
- Baandrup Jonas D., Markvardsen Lars H., Christian D., Schou U. K., Jensen J. L., Magnusson N. E., et al. (2011). Pressure load: The main factor for altered gene expression in right ventricular hypertrophy in chronic hypoxic rats. PLoS ONE 6 (1), e15859. 10.1371/journal.pone.0015859 - DOI - PMC - PubMed
-
- Baumgartner H., Bonhoeffer P., De Groot N. M., deHaan D., Deanfield J. E., Galie N., et al. (2010). ESC guidelines for the management of grown-up congenital heart disease (new version 2010): The task force on the management of grown-up congenital heart disease of the European society of cardiology (ESC). ESC Comm. Pract. Guidel. (CPG). Eur Heart J 31 (23), 2915–2957. 10.1093/eurheartj/ehq249 - DOI - PubMed
LinkOut - more resources
Full Text Sources

