Continuous separation of bacterial cells from large debris using a spiral microfluidic device
- PMID: 37576440
- PMCID: PMC10415021
- DOI: 10.1063/5.0159254
Continuous separation of bacterial cells from large debris using a spiral microfluidic device
Abstract
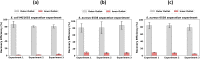
With the global increase in food exchange, rapid identification and enumeration of bacteria has become crucial for protecting consumers from bacterial contamination. Efficient analysis requires the separation of target particles (e.g., bacterial cells) from food and/or sampling matrices to prevent matrix interference with the detection and analysis of target cells. However, studies on the separation of bacteria-sized particles and defined particles, such as bacterial cells, from heterogeneous debris, such as meat swab suspensions, are limited. In this study, we explore the use of passive-based inertial microfluidics to separate bacterial cells from debris, such as fascia, muscle tissues, and cotton fibers, extracted from ground meat and meat swabs-a novel approach demonstrated for the first time. Our objective is to evaluate the recovery efficiency of bacterial cells from large debris obtained from ground meat and meat swab suspensions using a spiral microfluidic device. In this study, we establish the optimal flow rates and Dean number for continuous bacterial cell and debris separation and a methodology to determine the percentage of debris removed from the sample suspension. Our findings demonstrate an average recovery efficiency of 80% for bacterial cells separated from debris in meat swab suspensions, while the average recovery efficiency from ground beef suspensions was 70%. Furthermore, approximately 50% of the debris in the ground meat suspension were separated from bacterial cells.
© 2023 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures






References
-
- Payne M. J. and Kroll R. G., “Methods for the separation and concentration of bacteria from foods,” Trends Food Sci. Technol. 2, 315–319 (1991). 10.1016/0924-2244(91)90734-Z - DOI
-
- Clime L., Li K., Geissler M., Hoa X. D., Robideau G. P., Bilodeau G. J., and Veres T., “Separation and concentration of Phytophthora ramorum sporangia by inertial focusing in curving microfluidic flows,” Microfluid. Nanofluid. 21, 5 (2016). 10.1007/s10404-016-1844-9 - DOI
-
- Lee M.-L. and Yao D.-J., “The separation of microalgae using dean flow in a spiral microfluidic device,” Inventions 3, 40 (2018). 10.3390/inventions3030040 - DOI
LinkOut - more resources
Full Text Sources
