Multiparameter interferometric polarization-enhanced imaging differentiates carcinoma in situ from inflammation of the bladder: an ex vivo study
- PMID: 37576611
- PMCID: PMC10415042
- DOI: 10.1117/1.JBO.28.10.102907
Multiparameter interferometric polarization-enhanced imaging differentiates carcinoma in situ from inflammation of the bladder: an ex vivo study
Abstract
Significance: Successful differentiation of carcinoma in situ (CIS) from inflammation in the bladder is key to preventing unnecessary biopsies and enabling accurate therapeutic decisions. Current standard-of-care diagnostic imaging techniques lack the specificity needed to differentiate these states, leading to false positives.
Aim: We introduce multiparameter interferometric polarization-enhanced (MultiPIPE) imaging as a promising technology to improve the specificity of detection for better biopsy guidance and clinical outcomes.
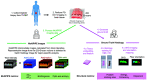
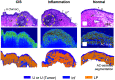
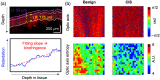
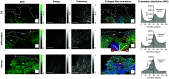
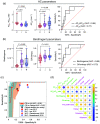
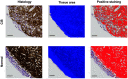
Approach: In this ex vivo study, we extract tissue attenuation-coefficient-based and birefringence-based parameters from MultiPIPE imaging data, collected with a bench-top system, to develop a classifier for the differentiation of benign and CIS tissues. We also analyze morphological features from second harmonic generation imaging and histology slides and perform imaging-to-morphology correlation analysis.
Results: MultiPIPE enhances specificity to differentiate CIS from benign tissues by nearly 20% and reduces the false-positive rate by more than four-fold over clinical standards. We also show that the MultiPIPE measurements correlate well with changes in morphological features in histological assessments.
Conclusions: The results of our study show the promise of MultiPIPE imaging to be used for better differentiation of bladder inflammation from flat tumors, leading to a fewer number of unnecessary procedures and shorter operating room (OR) time.
Keywords: bladder cancer; carcinoma in situ; inflammation; optical coherence tomography; polarization.
© 2023 The Authors.
Figures


Similar articles
-
New optical imaging technologies for bladder cancer: considerations and perspectives.J Urol. 2012 Aug;188(2):361-8. doi: 10.1016/j.juro.2012.03.127. Epub 2012 Jun 13. J Urol. 2012. PMID: 22698620 Free PMC article. Review.
-
Visualization of the basement membrane zone of the bladder by optical coherence tomography: feasibility of noninvasive evaluation of tumor invasion.Urology. 2008 Sep;72(3):677-81. doi: 10.1016/j.urology.2008.02.062. Epub 2008 May 2. Urology. 2008. PMID: 18455778
-
Multiphoton microscopy: a potential intraoperative tool for the detection of carcinoma in situ in human bladder.Arch Pathol Lab Med. 2015 Jun;139(6):796-804. doi: 10.5858/arpa.2014-0076-OA. Arch Pathol Lab Med. 2015. PMID: 26030249
-
Early detection of carcinoma in situ of the bladder: a comparative study of white light cystoscopy, narrow band imaging, 5-ALA fluorescence cystoscopy and 3-dimensional optical coherence tomography.J Urol. 2012 Mar;187(3):1063-70. doi: 10.1016/j.juro.2011.10.131. Epub 2012 Jan 15. J Urol. 2012. PMID: 22245332
-
Recent advances in optical imaging technologies for the detection of bladder cancer.Photodiagnosis Photodyn Ther. 2018 Dec;24:192-197. doi: 10.1016/j.pdpdt.2018.10.009. Epub 2018 Oct 10. Photodiagnosis Photodyn Ther. 2018. PMID: 30315954 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

