Exploration of Remarkably Potential Multitarget-Directed N-Alkylated-2-(substituted phenyl)-1 H-benzimidazole Derivatives as Antiproliferative, Antifungal, and Antibacterial Agents
- PMID: 37576624
- PMCID: PMC10413844
- DOI: 10.1021/acsomega.3c03530
Exploration of Remarkably Potential Multitarget-Directed N-Alkylated-2-(substituted phenyl)-1 H-benzimidazole Derivatives as Antiproliferative, Antifungal, and Antibacterial Agents
Abstract
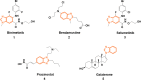
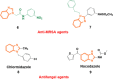
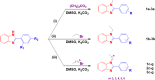
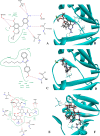
Improving lipophilicity for drugs to penetrate the lipid membrane and decreasing bacterial and fungal coinfections for patients with cancer pose challenges in the drug development process. Here, a series of new N-alkylated-2-(substituted phenyl)-1H-benzimidazole derivatives were synthesized and characterized by 1H and 13C NMR, FTIR, and HRMS spectrum analyses to address these difficulties. All the compounds were evaluated for their antiproliferative, antibacterial, and antifungal activities. Results indicated that compound 2g exhibited the best antiproliferative activity against the MDA-MB-231 cell line and also displayed significant inhibition at minimal inhibitory concentration (MIC) values of 8, 4, and 4 μg mL-1 against Streptococcus faecalis, Staphylococcus aureus, and methicillin-resistant Staphylococcus aureus compared with amikacin. The antifungal data of compounds 1b, 1c, 2e, and 2g revealed their moderate activities toward Candida albicans and Aspergillus niger, with MIC values of 64 μg mL-1 for both strains. Finally, the molecular docking study found that 2g interacted with crucial amino acids in the binding site of complex dihydrofolate reductase with nicotinamide adenine dinucleotide phosphate.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
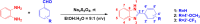


References
-
- Satija G.; Sharma B.; Madan A.; Iqubal A.; Shaquiquzzaman M.; Akhter M.; Parvez S.; Khan M. A.; Alam M. M. Benzimidazole based derivatives as anticancer agents: Structure activity relationship analysis for various targets. J. Heterocycl. 2022, 59, 22–66. 10.1002/jhet.4355. - DOI
-
- Ibrahim H. A.; Refaat H. M. Versatile mechanisms of 2-substituted benzimidazoles in targeted cancer therapy. Future J. Pharm. Sci. 2020, 6, 1–20. 10.1186/s43094-020-00048-8. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

