Grafting Strategies of Oxidation-Prone Coiled-Coil Peptides for Protein Capture in Bioassays: Impact of Orientation and the Oxidation State
- PMID: 37576632
- PMCID: PMC10413464
- DOI: 10.1021/acsomega.3c02172
Grafting Strategies of Oxidation-Prone Coiled-Coil Peptides for Protein Capture in Bioassays: Impact of Orientation and the Oxidation State
Abstract
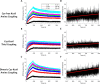
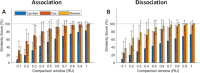
Many biomedical and biosensing applications require functionalization of surfaces with proteins. To this end, the E/K coiled-coil peptide heterodimeric system has been shown to be advantageous. First, Kcoil peptides are covalently grafted onto a given surface. Ecoil-tagged proteins can then be non-covalently captured via a specific interaction with their Kcoil partners. Previously, oriented Kcoil grafting was achieved via thiol coupling, using a unique Kcoil with a terminal cysteine residue. However, cysteine-terminated Kcoil peptides are hard to produce, purify, and oxidize during storage. Indeed, they tend to homodimerize and form disulfide bonds via oxidation of their terminal thiol group, making it impossible to later graft them on thiol-reactive surfaces. Kcoil peptides also contain multiple free amine groups, available for covalent coupling through carbodiimide chemistry. Grafting Kcoil peptides on surfaces via amine coupling would thus guarantee their immobilization regardless of their terminal cysteine's oxidation state, at the expense of the control over their orientation. In this work, we compare Kcoil grafting strategies for the subsequent capture of Ecoil-tagged proteins, for applications such as surface plasmon resonance (SPR) biosensing and cell culture onto protein-decorated substrates. We compare the "classic" thiol coupling of cysteine-terminated Kcoil peptides to the amine coupling of (i) monomeric Kcoil and (ii) dimeric Kcoil-Kcoil linked by a disulfide bond. We have observed that SPR biosensing performances relying on captured Ecoil-tagged proteins were similar for amine-coupled dimeric Kcoil-Kcoil and thiol-coupled Kcoil peptides, at the expense of higher Ecoil-tagged protein consumption. For cell culture applications, Ecoil-tagged growth factors captured on amine-coupled monomeric Kcoil signaled through cell receptors similarly to those captured on thiol-coupled Kcoil peptides. Altogether, while oriented thiol coupling of cysteine-terminated Kcoil peptides remains the most reliable and versatile platform for Ecoil-tagged protein capture, amine coupling of Kcoil peptides, either monomeric or dimerized through a cysteine bond, can offer a good alternative when the challenges and costs associated with the production of monomeric cysteine-tagged Kcoil are too dissuasive for the application.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Tutar R.; Motealleh A.; Khademhosseini A.; Kehr N. S. Functional Nanomaterials on 2D Surfaces and in 3D Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2019, 29, 1904344. 10.1002/adfm.201904344. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

