Roles of DNA Target in Cancer Cell-Selective Cytotoxicity by Dicopper Complexes with DNA Target/Ligand Conjugates
- PMID: 37576680
- PMCID: PMC10413468
- DOI: 10.1021/acsomega.3c03387
Roles of DNA Target in Cancer Cell-Selective Cytotoxicity by Dicopper Complexes with DNA Target/Ligand Conjugates
Abstract
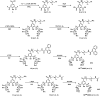
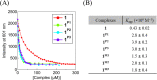
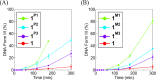
The DNA target/ligand conjugates (HLX, X = Pn and Mn, n = 1-3) were synthesized where various lengths of -CONH(CH2CH2O)nCH2CH2NHCO- linkers with a 9-phenanthrenyl (P) or methyl (M) terminal as DNA targets replace the methyl group of 2,6-di(amide-tether cyclen)-p-cresol ligand (HL). DNA binding, DNA cleavage, cellular uptake, and cytotoxicity of [Cu2(μ-OH)(LX)](ClO4)2 (1X) are examined and compared with those of [Cu2(μ-OH)(L)](ClO4)2 (1) to clarify roles of DNA targets. Upon reaction of 1X with H2O2, μ-1,1-O2H complexes are formed for DNA cleavage. 1P1, 1P2, and 1P3 are 22-, 11-, 3-fold more active for conversion of Form II to III in the cleavage of supercoiled plasmid DNA with H2O2 than 1, where the short P-linker may fix a dicopper moiety within a small number of base pairs to facilitate DNA double-strand breaks (dsb). This enhances the proapoptotic activity of 1P1, 1P2, and 1P3, which are 30-, 12-, and 9.9-fold cytotoxic against HeLa cells than 1. DNA dsb and cytotoxicity are 44% correlated in 1P1-3 but 5% in 1M1-3, suggesting specific DNA binding of P-linkers and nonspecific binding of M-linkers in biological cells. 1P1-3 exert cancer cell-selective cytotoxicity against lung and pancreas cancer and normal cells where the short P-linker enhances the selectivity, but 1M1-3 do not. Intracellular visualization, apoptosis assay, and caspase activity assay clarify mitochondrial apoptosis caused by 1P1-3. The highest cancer cell selectivity of 1P1 may be enabled by the short P-linker promoting dsb of mitochondrial DNA with H2O2 increased by mitochondrial dysfunction in cancer cells.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

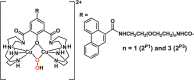

References
-
- Boulikas T.; Pantos A.; Bellis E.; Christofis P. Designing platinum compounds in cancer: structures and mechanisms. Cancer Ther. 2007, 5, 537–583.
-
- Liu L. V.; Bell C. B. III; Wong S. D.; Wilson S. A.; Kwak Y.; Chow M. S.; Zhao J.; Hodgson K. O.; Hedman B.; Solomon E. I. Definition of the intermediates and mechanism of the anticancer drug bleomycin using nuclear resonance vibrational spectroscopy and related methods. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 22419–22424. 10.1073/pnas.1016323107. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

