Effects of ethanol washing and storage duration on primary culture of stem cells from human exfoliated deciduous teeth
- PMID: 37576800
- PMCID: PMC10415792
- DOI: 10.1016/j.jobcr.2023.06.005
Effects of ethanol washing and storage duration on primary culture of stem cells from human exfoliated deciduous teeth
Abstract
Purpose: Since the oral environment harbors various microorganisms, the removal of contaminants during the primary culture process of stem cells from human exfoliated deciduous teeth (SHEDs) is very important. We investigated optimal methods for primary culture of SHEDs with minimal contamination rates.
Materials and methods: Three different storage conditions for deciduous teeth were utilized:1) storing teeth in Hank's Balanced Salt Solution (HBSS) with 3% penicillin and streptomycin (P/S), 2) storing teeth in HBSS with 3% antibiotics and antimycotics (A-A), and 3) storing teeth in HBSS with A-A, and additional washing with 70% ethanol just before primary culture of dental pulp. In addition, the storage time from the extraction of teeth to the primary culture was measured.
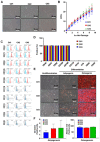
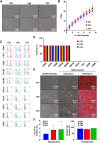
Results: The contamination rates were about 70% for HBSS with P/S, 40% for HBSS with A-A, and less than 10% for HBSS with A-A and additional washing with 70% ethanol. When the primary culture was conducted within 12 h after teeth extraction, the contamination rate was the lowest in all conditions. Furthermore, when the teeth were delivered in HBSS with A-A and an additional 70% ethanol washing was performed, the contamination rate was 0% until 48 h after teeth extraction. Ethanol washing had little effect on the cellular characteristics and stemness of SHEDs, including their morphology, growth rate, expression of surface markers, and differentiation potential.
Conclusions: We suggested that both delivering teeth in HBSS with A-A and additional 70% ethanol washing are critical considerations for the successful culture of SHEDs without contamination.
Keywords: Antibiotics; Contamination; Ethanol; Primary culture; Stem cells from human exfoliated deciduous teeth.
© 2023 Published by Elsevier B.V. on behalf of Craniofacial Research Foundation.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Nakashima M., Iohara K., Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20:435–440. - PubMed
LinkOut - more resources
Full Text Sources

