State-of-the-Science of human papillomavirus vaccination in women with human immunodeficiency Virus: Summary of a scientific workshop
- PMID: 37576844
- PMCID: PMC10413150
- DOI: 10.1016/j.pmedr.2023.102331
State-of-the-Science of human papillomavirus vaccination in women with human immunodeficiency Virus: Summary of a scientific workshop
Abstract
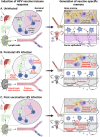
The burden of cervical cancer is disproportionately distributed globally, with the vast majority of cases occurring in low- and middle-income countries. Women with human immunodeficiency virus (HIV) (WWH) are at increased risk of human papillomavirus (HPV) infection and cervical cancer as compared to HIV-negative individuals. HPV vaccination remains a priority in regions with a high burden of cervical cancer and high HIV prevalence. With HPV vaccines becoming more accessible, optimal use beyond the initial World Health Organization-recommended target population of 9 to 14-year-old girls is an important question. In March 2022, a group of experts in epidemiology, immunology, and vaccinology convened to discuss the state-of-the-science of HPV vaccination in WWH. This report summarizes the proceedings: review of HIV epidemiology and its intersection with cervical cancer burden, immunology, HPV vaccination including reduced-dose schedules and experience with other vaccines in people with HIV (PWH), HPV vaccination strategies and knowledge gaps, and outstanding research questions. Studies of HPV vaccine effectiveness among WWH, including duration of protection, are limited. Until data from ongoing research is available, the current recommendation for WWH remains for a multi-dose HPV vaccination regimen. A focus of the discussion included the potential impact of HIV acquisition following HPV vaccination. With no data currently existing for HPV vaccines and limited information from non-HPV vaccines, this question requires further research. Implementation research on optimal HPV vaccine delivery approaches for WWH and other priority populations is also urgently needed.
Keywords: Epidemiology; HPV vaccine; Human papillomavirus (HPV); Immunization; Immunology; Prevention; Vaccination strategies; human immunodeficiency virus (HIV); women with HIV (WWH).
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: AMD reports participation in advisory boards for Sanofi, Bioaster, Speranza and ACM Biolabs, and research grants from GlaxoSmithKline, Moderna, and Roche, outside the submitted work. ABM reports personal fees from Merck & Co. global advisory board honorarium, outside the submitted work. GL is an employee of Merck & Co. NM is a recipient of an Investigator Initiated Grant evaluating ‘Immunogenicity of HPV vaccine among HIV infected adolescents’, outside the submitted work. JMP reports grants and personal fees from Merck & Co., from Vir Biotechnologies, from Antiva Biosciences, from Roche Diagnostics, and other from Virion Therapeutics, outside the submitted work. JS reports participation to DSMB for NCT04508309 and the India/IARC Gardasil trial. SAM reports grants from Bill & Melinda Gates Foundation, Pfizer, GlaxoSmithKline, Minervax and Sanofi, outside the submitted work. SP reports personal fees from Merck & Co. global advisory board honorarium, and personal fees from Sanofi, Inovio, Merck & Co., Janssen, Moderna, Valneva, Codagenix, Pfizer, VaxArt, Meissa, Vaxinnity, Rational, and AstraZeneca, outside the submitted work. RB reports grants from National Institutes of Health and Bill & Melinda Gates Foundation and non-financial support from Regeneron Pharmaceutical, outside the submitted work. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Amanna I.J., Carlson N.E., Slifka M.K. Duration of humoral immunity to common viral and vaccine antigens. New Eng J Med. 2007;357(19):1903–1915. - PubMed
-
- Appay V., Sauce D., Kelleher A.D. In: Handbook of Immunosenescence: Basic Understanding and Clinical Implications. Fulop T., Franceschi C., Hirokawa K., Pawelec G., editors. Springer International Publishing; Cham: 2019. HIV Infection as a Model of Accelerated Immunosenescence; pp. 1961–1989.
-
- Bruni L., Albero G., Serrano B., Mena M., Gómez D., Muñoz J., Bosch F.X., de Sanjosé S. Barcelona, Spain; ICO HPV Information Centre: 2021. Human Papillomavirus and Related Diseases in the World—Summary Report 22 October 2021.

