Artificial cells eavesdropping on HepG2 cells
- PMID: 37577001
- PMCID: PMC10415741
- DOI: 10.1098/rsfs.2023.0007
Artificial cells eavesdropping on HepG2 cells
Abstract
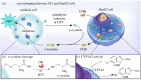
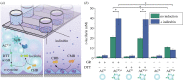
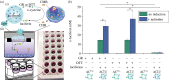
Cellular communication is a fundamental feature to ensure the survival of cellular assemblies, such as multicellular tissue, via coordinated adaption to changes in their surroundings. Consequently, the development of integrated semi-synthetic systems consisting of artificial cells (ACs) and mammalian cells requires feedback-based interactions. Here, we illustrate that ACs can eavesdrop on HepG2 cells focusing on the activity of cytochrome P450 1A2 (CYP1A2), an enzyme from the cytochrome P450 enzyme family. Specifically, d-cysteine is sent as a signal from the ACs via the triggered reduction of disulfide bonds. Simultaneously, HepG2 cells enzymatically convert 2-cyano-6-methoxybenzothiazole into 2-cyano-6-hydroxybenzothiazole that is released in the extracellular space. d-Cysteine and 2-cyano-6-hydroxybenzothiazole react to form d-luciferin. The ACs respond to this signal by converting d-luciferin into luminescence due to the presence of encapsulated luciferase in the ACs. As a result, the ACs can eavesdrop on the mammalian cells to evaluate the level of hepatic CYP1A2 function.
Keywords: artificial cell; cell mimicry; cellular communication; cytochrome P450.
© 2023 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
-
- Belluati A, Thamboo S, Najer A, Maffeis V, Planta C, Craciun I, Palivan CG, Meier W. 2020. Multicompartment polymer vesicles with artificial organelles for signal-triggered cascade reactions including cytoskeleton formation. Adv. Funct. Mater. 30, 2002949. ( 10.1002/adfm.202002949) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
