Intramuscular vs Intravenous SARS-CoV-2 Neutralizing Antibody Sotrovimab for Treatment of COVID-19 (COMET-TAIL): A Randomized Noninferiority Clinical Trial
- PMID: 37577112
- PMCID: PMC10414803
- DOI: 10.1093/ofid/ofad354
Intramuscular vs Intravenous SARS-CoV-2 Neutralizing Antibody Sotrovimab for Treatment of COVID-19 (COMET-TAIL): A Randomized Noninferiority Clinical Trial
Abstract
Background: Convenient administration of coronavirus disease 2019 (COVID-19) treatment in community settings is desirable. Sotrovimab is a pan-sarbecovirus dual-action monoclonal antibody formulated for intravenous (IV) or intramuscular (IM) administration for early treatment of mild/moderate COVID-19.
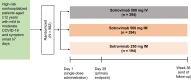
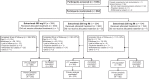
Method: This multicenter phase 3 study based on a randomized open-label design tested the noninferiority of IM to IV administration according to an absolute noninferiority margin of 3.5%. From June to August 2021, patients aged ≥12 years with COVID-19, who were neither hospitalized nor receiving supplemental oxygen but were at high risk for progression, were randomized 1:1:1 to receive sotrovimab as a single 500-mg IV infusion or a 500- or 250-mg IM injection. The primary composite endpoint was progression to (1) all-cause hospitalization for >24 hours for acute management of illness or (2) all-cause death through day 29.
Results: Sotrovimab 500 mg IM was noninferior to 500 mg IV: 10 (2.7%) of 376 participants vs 5 (1.3%) of 378 met the primary endpoint, respectively (absolute adjusted risk difference, 1.06%; 95% CI, -1.15% to 3.26%). The 95% CI upper limit was lower than the prespecified noninferiority margin of 3.5%. The 250-mg IM group was discontinued early because of the greater proportion of hospitalizations vs the 500-mg groups. Serious adverse events occurred in <1% to 2% of participants across groups. Four participants experienced serious disease-related events and died (500 mg IM, 2/393, <1%; 250 mg IM, 2/195, 1%).
Conclusions: Sotrovimab 500-mg IM injection was well tolerated and noninferior to IV administration. IM administration could expand outpatient treatment access for COVID-19.
Clinical trials registration: ClinicalTrials.gov: NCT04913675.
Keywords: COMET-TAIL; COVID-19 treatment; intramuscular administration; neutralizing monoclonal antibody; sotrovimab.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. E. S., J. A., A. F., Y. G.-R., R. H., E. J., J. M., and N. P. report acting as trial investigators for Vir Biotechnology and receiving nonfinancial support from Vir Biotechnology during the conduct of the study. A. K. reports acting as a trial investigator for Vir Biotechnology and receiving nonfinancial support from Vir Biotechnology during the conduct of the study; grants and consulting fees from Gilead Biosciences; and clinical trial payments from Regeneron, Vir Biotechnology, GSK, and Gilead Biosciences. A. K. also reports serving on data safety monitoring or advisory boards for Gilead Biosciences. E. S. reports acting as a trial investigator for Vir Biotechnology and receiving nonfinancial support from Vir Biotechnology during the conduct of the study and research support from AbbVie, Eli Lilly, Otsuka, Eisai, and Ironshore. E. S. also reports serving on speaker bureaus for Janssen, Teva, and AbbVie. D. C., M. L. A., S. P., S. C., E. M., J. E. S., W. W. Y., E. L. A., and L. A. G. are employees of Vir Biotechnology and report stock ownership in Vir Biotechnology and third-party funding from GSK to Vir Biotechnology for the submitted work. P. S. P. is an employee of Vir Biotechnology and reports stock ownership in Vir Biotechnology, third-party funding from GSK to Vir Biotechnology for the submitted work, and patents pending for sotrovimab for the treatment of COVID-19. D. K. H. was an employee of Vir Biotechnology and held stock in Vir Biotechnology at the time of the study; he is currently an employee of Janssen Pharmaceutical Companies of Johnson & Johnson and reports stock ownership in Johnson & Johnson. D. I., A. S., D. A., A. P., A. N., N. N., and Q. W. are employees of GSK and report stock ownership in GSK.
Figures
References
-
- Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early COVID-19 treatment with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

