Delayed and Attenuated Antibody Responses to Coronavirus Disease 2019 Vaccination With Poor Cross-Variant Neutralization in Solid-Organ Transplant Recipients-A Prospective Longitudinal Study
- PMID: 37577118
- PMCID: PMC10414143
- DOI: 10.1093/ofid/ofad369
Delayed and Attenuated Antibody Responses to Coronavirus Disease 2019 Vaccination With Poor Cross-Variant Neutralization in Solid-Organ Transplant Recipients-A Prospective Longitudinal Study
Abstract
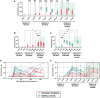
Background: Therapeutically immunosuppressed transplant recipients exhibit attenuated responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines. To elucidate the kinetics and variant cross-protection of vaccine-induced antibodies in this population, we conducted a prospective longitudinal study in heart and lung transplant recipients receiving the SARS-CoV-2 messenger RNA (mRNA) 3-dose vaccination series.
Methods: We measured longitudinal serum antibody and neutralization responses against the ancestral and major variants of SARS-CoV-2 in SARS-CoV-2-uninfected lung (n = 18) and heart (n = 17) transplant recipients, non-lung-transplanted patients with cystic fibrosis (n = 7), and healthy controls (n = 12) before, during, and after the primary mRNA vaccination series.
Results: Among healthy controls, strong anti-spike responses arose immediately following vaccination and displayed cross-neutralization against all variants. In contrast, among transplant recipients, after the first 2 vaccine doses, increases in antibody concentrations occurred gradually, and cross-neutralization was completely absent against the Omicron B.1.1.529 variant. However, most (73%) of the transplant recipients had a significant response to the third vaccine dose, reaching levels comparable to those of healthy controls, with improved but attenuated neutralization of immune evasive variants, particularly Beta, Gamma, and Omicron. Responses in non-lung-transplanted patients with cystic fibrosis paralleled those in healthy controls.
Conclusions: In this prospective, longitudinal analysis of variant-specific antibody responses, lung and heart transplant recipients display delayed and defective responses to the first 2 SARS-CoV-2 vaccine doses but significantly augmented responses to a third dose. Gaps in antibody-mediated immunity among transplant recipients are compounded by decreased neutralization against Omicron variants, leaving many patients with substantially weakened immunity against currently circulating variants.
Keywords: COVID-19 vaccination; cross-variant neutralization; cystic fibrosis; longitudinal antibody responses; solid-organ transplant recipients.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

