Extracting accurate infrared lineshapes from weak vibrational probes at low concentrations
- PMID: 37577166
- PMCID: PMC10416016
- DOI: 10.1016/j.mex.2023.102309
Extracting accurate infrared lineshapes from weak vibrational probes at low concentrations
Abstract
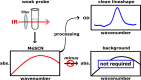
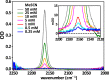
Fourier-transform infrared (FTIR) spectroscopy using vibrational probes is an ideal tool to detect changes in structure and local environments within biological molecules. However, challenges arise when dealing with weak infrared probes, such as thiocyanates, due to their inherent low signal strengths and overlap with solvent bands. In this protocol we demonstrate:•A streamlined approach for the precise extraction of weak infrared absorption lineshapes from a strong solvent background.•A protocol combining a spectral filter, background modeling, and subtraction.•Our methodology successfully extracts the CN stretching mode peak from methyl thiocyanate at remarkably low concentrations (0.25 mM) in water, previously a challenge for FTIR spectroscopy.This approach offers valuable insights and tools for more accurate FTIR measurements using weak vibrational probes. This enhanced precision can potentially enable new approaches to enhance our understanding of protein structure and dynamics in solution.
Keywords: Analysis of weak vibrational probes; Data processing; FTIR; Protein structure; Spectroscopy; Thiocyanate; Vibrational modes.
© 2023 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- L. Banci, Metallomics and the Cell. Metallomics Cell 2013.
-
- Cavanagh M.C., Young R.M., Schwartz B.J. The roles of the solute and solvent cavities in charge-transfer-to-solvent dynamics: ultrafast studies of potasside and sodide in diethyl ether. J. Chem. Phys. 2008;129(13) - PubMed
-
- Okur H.I., Kherb J., Cremer P.S. Cations bind only weakly to amides in aqueous solutions. J. Am. Chem. Soc. 2013;135(13):5062–5067. - PubMed
-
- Barth A., Zscherp C. What vibrations tell us about proteins. Q. Rev. Biophys. 2002;35(4):369–430. - PubMed
LinkOut - more resources
Full Text Sources

