Portal Hypertension in Nonalcoholic Fatty Liver Disease: Challenges and Paradigms
- PMID: 37577237
- PMCID: PMC10412712
- DOI: 10.14218/JCTH.2023.00029
Portal Hypertension in Nonalcoholic Fatty Liver Disease: Challenges and Paradigms
Abstract
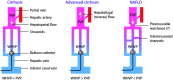
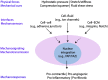
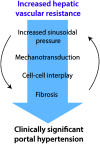
Portal hypertension in cirrhosis is defined as an increase in the portal pressure gradient (PPG) between the portal and hepatic veins and is traditionally estimated by the hepatic venous pressure gradient (HVPG), which is the difference in pressure between the free-floating and wedged positions of a balloon catheter in the hepatic vein. By convention, HVPG≥10 mmHg indicates clinically significant portal hypertension, which is associated with adverse clinical outcomes. Nonalcoholic fatty liver disease (NAFLD) is a common disorder with a heterogeneous clinical course, which includes the development of portal hypertension. There is increasing evidence that portal hypertension in NAFLD deserves special considerations. First, elevated PPG often precedes fibrosis in NAFLD, suggesting a bidirectional relationship between these pathological processes. Second, HVPG underestimates PPG in NAFLD, suggesting that portal hypertension is more prevalent in this condition than currently believed. Third, cellular mechanoresponses generated early in the pathogenesis of NAFLD provide a mechanistic explanation for the pressure-fibrosis paradigm. Finally, a better understanding of liver mechanobiology in NAFLD may aid in the development of novel pharmaceutical targets for prevention and management of this disease.
Keywords: Hepatic vascular resistance; Hepatic venous pressure gradient; Mechanobiology; Mechanotransduction; Portal venous pressure; Sinusoidal microcirculation.
© 2023 Authors.
Conflict of interest statement
GB has been an associate editor of the Journal of Clinical and Translational Hepatology since 2020. EKM and PP have no conflict of interests related to this publication.
Figures

Similar articles
-
Portal hypertension in patients with nonalcoholic fatty liver disease: Current knowledge and challenges.World J Gastroenterol. 2024 Jan 28;30(4):290-307. doi: 10.3748/wjg.v30.i4.290. World J Gastroenterol. 2024. PMID: 38313235 Free PMC article. Review.
-
Nonalcoholic fatty liver disease and portal hypertension.Explor Med. 2020;1:149-169. doi: 10.37349/emed.2020.00011. Epub 2020 Jun 29. Explor Med. 2020. PMID: 32685936 Free PMC article.
-
[Study on the correlation between PPG and HVPG in patients with portal hypertension].Zhonghua Gan Zang Bing Za Zhi. 2022 Jul 20;30(7):722-727. doi: 10.3760/cma.j.cn501113-20200603-00291. Zhonghua Gan Zang Bing Za Zhi. 2022. PMID: 36038341 Chinese.
-
A computational model of the hepatic circulation applied to analyze the sensitivity of hepatic venous pressure gradient (HVPG) in liver cirrhosis.J Biomech. 2017 Dec 8;65:23-31. doi: 10.1016/j.jbiomech.2017.09.023. Epub 2017 Oct 6. J Biomech. 2017. PMID: 29042056
-
Hepatic venous pressure gradient measurement: time to learn!Indian J Gastroenterol. 2008 Mar-Apr;27(2):74-80. Indian J Gastroenterol. 2008. PMID: 18695309 Review.
Cited by
-
Role of noninvasive tests in the prognostication of metabolic dysfunction-associated steatotic liver disease.Clin Mol Hepatol. 2025 Feb;31(Suppl):S51-S75. doi: 10.3350/cmh.2024.0246. Epub 2024 Jun 27. Clin Mol Hepatol. 2025. PMID: 38934108 Free PMC article. Review.
-
The role of anoctamin 1 in liver disease.J Cell Mol Med. 2024 May;28(9):e18320. doi: 10.1111/jcmm.18320. J Cell Mol Med. 2024. PMID: 38685684 Free PMC article. Review.
-
Portal hypertension in patients with nonalcoholic fatty liver disease: Current knowledge and challenges.World J Gastroenterol. 2024 Jan 28;30(4):290-307. doi: 10.3748/wjg.v30.i4.290. World J Gastroenterol. 2024. PMID: 38313235 Free PMC article. Review.
-
Hepatic microcirculatory disturbance in liver diseases: intervention with traditional Chinese medicine.Front Pharmacol. 2024 Jul 23;15:1399598. doi: 10.3389/fphar.2024.1399598. eCollection 2024. Front Pharmacol. 2024. PMID: 39108760 Free PMC article. Review.
-
Advances in the diagnosis and management of clinically significant portal hypertension in cirrhosis: A narrative review.World J Hepatol. 2025 Jun 27;17(6):104761. doi: 10.4254/wjh.v17.i6.104761. World J Hepatol. 2025. PMID: 40606915 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
