New insight into the virulence and inflammatory response of Staphylococcus aureus strains isolated from diabetic foot ulcers
- PMID: 37577369
- PMCID: PMC10416727
- DOI: 10.3389/fcimb.2023.1234994
New insight into the virulence and inflammatory response of Staphylococcus aureus strains isolated from diabetic foot ulcers
Abstract
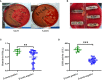
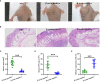
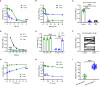
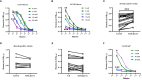
Staphylococcus aureus strains isolated from diabetic foot ulcers (DFUs) have less virulence, but still cause severe infections. Furthermore, hypovirulent S. aureus strains appear to be localized in the deep tissues of diabetic foot osteomyelitis, indicating that the unique environment within DFUs affects the pathogenicity of S. aureus. In this study, the cell-free culture medium (CFCM) of S. aureus strains isolated from DFUs exhibited higher cytotoxicity to human erythrocytes than those isolated from non-diabetic patients with sepsis or wounds. Among these S. aureus strains isolated from DFUs, β-toxin negative strains have less virulence than β-toxin positive strains, but induced a higher expression of inflammatory cytokines. Our study and previous studies have shown that the synergistic effect of phenol-soluble modulin α and β-toxin contributes to the higher hemolytic activity of β-toxin positive strains. However, lysis of human erythrocytes by the CFCM of β-toxin negative strains was greatly inhibited by an autolysin inhibitor, sodium polyanethole sulfonate (SPS). A high level of glucose greatly reduced the hemolytic activity of S. aureus, but promoted the expression of interleukin-6 (IL-6) in human neutrophils. However, 5 mM glucose or glucose-6-phosphate (G6P) increased the hemolytic activity of SA118 (a β-toxin negative strain) isolated from DFUs. Additionally, patients with DFUs with growth of S. aureus had lower level of serum IL-6 than those with other bacteria, and the CFCM of S. aureus strains significantly reduced lipopolysaccharide-induced IL-6 expression in human neutrophils. Therefore, the virulence and inflammatory response of S. aureus strains isolated from DFUs are determined by the levels of glucose and its metabolites, which may explain why it is the predominant bacteria isolated from DFUs.
Keywords: Staphylococcus aureus; diabetic foot ulcers; inflammatory response; virulence; β-toxin.
Copyright © 2023 Wu, Chen, Wang, Huang, Wang and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Cassat J. E., Hammer N. D., Campbell J. P., Benson M. A., Perrien D. S., Mrak L. N., et al. (2013). A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host Microbe 13, 759–772. doi: 10.1016/j.chom.2013.05.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

