This is a preprint.
Tmem263 deletion disrupts the GH/IGF-1 axis and causes dwarfism and impairs skeletal acquisition
- PMID: 37577461
- PMCID: PMC10418210
- DOI: 10.1101/2023.08.02.551694
Tmem263 deletion disrupts the GH/IGF-1 axis and causes dwarfism and impairs skeletal acquisition
Update in
- This article has been published with doi: 10.7554/eLife.90949.3 doi: 10.7554/eLife.90949.3
-
Tmem263 deletion disrupts the GH/IGF-1 axis and causes dwarfism and impairs skeletal acquisition.Elife. 2024 Jan 19;12:RP90949. doi: 10.7554/eLife.90949. Elife. 2024. PMID: 38241182 Free PMC article.
Abstract
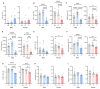
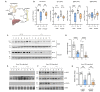
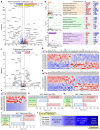
Genome-wide association studies (GWAS) have identified a large number of candidate genes believed to affect longitudinal bone growth and bone mass. One of these candidate genes, TMEM263, encodes a poorly characterized plasma membrane protein. Single nucleotide polymorphisms in TMEM263 are associated with bone mineral density in humans and mutations are associated with dwarfism in chicken and severe skeletal dysplasia in at least one human fetus. Whether this genotype-phenotype relationship is causal, however, remains unclear. Here, we determine whether and how TMEM263 is required for postnatal growth. Deletion of the Tmem263 gene in mice causes severe postnatal growth failure, proportional dwarfism, and impaired skeletal acquisition. Mice lacking Tmem263 show no differences in body weight within the first two weeks of postnatal life. However, by P21 there is a dramatic growth deficit due to a disrupted GH/IGF-1 axis, which is critical for longitudinal bone growth. Tmem263-null mice have low circulating IGF-1 levels and pronounced reductions in bone mass and growth plate length. The low serum IGF-1 in Tmem263-null mice is associated with reduced hepatic GH receptor (GHR) expression and GH-induced JAK2/STAT5 signaling. A deficit in GH signaling dramatically alters GH-regulated genes and feminizes the liver transcriptome of Tmem263-null male mice, with their expression profile resembling a wild-type female, hypophysectomized male, and Stat5b-null male mice. Collectively, our data validates the causal role for Tmem263 in regulating postnatal growth and raises the possibility that rare mutations or variants of TMEM263 may potentially cause GH insensitivity and impair linear growth.
Keywords: GHR; IGF-1; Laron dwarfism; growth hormone; growth hormone insensitivity; skeletal dysplasia.
Conflict of interest statement
COMPETING INTERESTS We declare that none of the authors has a conflict of interest.
Figures



References
-
- Estrada K., Styrkarsdottir U., Evangelou E., Hsu Y. H., Duncan E. L., Ntzani E. E., Oei L., Albagha O. M., Amin N., Kemp J. P., Koller D. L., Li G., Liu C. T., Minster R. L., Moayyeri A., Vandenput L., Willner D., Xiao S. M., Yerges-Armstrong L. M., Zheng H. F., Alonso N., Eriksson J., Kammerer C. M., Kaptoge S. K., Leo P. J., Thorleifsson G., Wilson S. G., Wilson J. F., Aalto V., Alen M., Aragaki A.K., Aspelund T., Center J. R., Dailiana Z., Duggan D. J., Garcia M., Garcia-Giralt N., Giroux S., Hallmans G., Hocking L. J., Husted L. B., Jameson K. A., Khusainova R., Kim G. S., Kooperberg C., Koromila T., Kruk M., Laaksonen M., Lacroix A. Z., Lee S. H., Leung P. C., Lewis J. R., Masi L., Mencej-Bedrac S., Nguyen T. V., Nogues X., Patel M. S., Prezelj J., Rose L. M., Scollen S., Siggeirsdottir K., Smith A. V., Svensson O., Trompet S., Trummer O., van Schoor N. M., Woo J., Zhu K., Balcells S., Brandi M. L., Buckley B. M., Cheng S., Christiansen C., Cooper C., Dedoussis G., Ford I., Frost M., Goltzman D., Gonzalez-Macias J., Kahonen M., Karlsson M., Khusnutdinova E., Koh J. M., Kollia P., Langdahl B. L., Leslie W. D., Lips P., Ljunggren O., Lorenc R. S., Marc J., Mellstrom D., Obermayer-Pietsch B., Olmos J. M., Pettersson-Kymmer U., Reid D. M., Riancho J. A., Ridker P. M., Rousseau F., Slagboom P. E., Tang N. L., Urreizti R., Van Hul W., Viikari J., Zarrabeitia M. T., Aulchenko Y. S., Castano-Betancourt M., Grundberg E., Herrera L., Ingvarsson T., Johannsdottir H., Kwan T., Li R., Luben R., Medina-Gomez C., Palsson S. T., Reppe S., Rotter J. I., Sigurdsson G., van Meurs J. B., Verlaan D., Williams F. M., Wood A. R., Zhou Y., Gautvik K. M., Pastinen T., Raychaudhuri S., Cauley J. A., Chasman D. I., Clark G. R., Cummings S. R., Danoy P., Dennison E. M., Eastell R., Eisman J. A., Gudnason V., Hofman A., Jackson R. D., Jones G., Jukema J. W., Khaw K. T., Lehtimaki T., Liu Y., Lorentzon M., McCloskey E., Mitchell B. D., Nandakumar K., Nicholson G. C., Oostra B. A., Peacock M., Pols H. A., Prince R. L., Raitakari O., Reid I. R., Robbins J., Sambrook P. N., Sham P. C., Shuldiner A. R., Tylavsky F. A., van Duijn C. M., Wareham N. J., Cupples L. A., Econs M. J., Evans D. M., Harris T. B., Kung A. W., Psaty B. M., Reeve J., Spector T. D., Streeten E. A., Zillikens M. C., Thorsteinsdottir U., Ohlsson C., Karasik D., Richards J. B., Brown M. A., Stefansson K., Uitterlinden A. G., Ralston S. H., Ioannidis J. P., Kiel D. P., and Rivadeneira F. (2012) Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 44, 491–501 - PMC - PubMed
-
- Kemp J. P., Morris J. A., Medina-Gomez C., Forgetta V., Warrington N. M., Youlten S. E., Zheng J., Gregson C. L., Grundberg E., Trajanoska K., Logan J. G., Pollard A. S., Sparkes P. C., Ghirardello E. J., Allen R., Leitch V. D., Butterfield N. C., Komla-Ebri D., Adoum A. T., Curry K. F., White J. K., Kussy F., Greenlaw K. M., Xu C., Harvey N. C., Cooper C., Adams D. J., Greenwood C. M. T., Maurano M. T., Kaptoge S., Rivadeneira F., Tobias J. H., Croucher P. I., Ackert-Bicknell C. L., Bassett J. H. D., Williams G. R., Richards J. B., and Evans D. M. (2017) Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis. Nat Genet 49, 1468–1475 - PMC - PubMed
-
- Zhao L. J., Liu X. G., Liu Y. Z., Liu Y. J., Papasian C. J., Sha B. Y., Pan F., Guo Y. F., Wang L., Yan H., Xiong D. H., Tang Z. H., Yang T. L., Chen X. D., Guo Y., Li J., Shen H., Zhang F., Lei S. F., Recker R. R., and Deng H. W. (2010) Genome-wide association study for femoral neck bone geometry. J Bone Miner Res 25, 320–329 - PMC - PubMed
-
- Styrkarsdottir U., Stefansson O. A., Gunnarsdottir K., Thorleifsson G., Lund S. H., Stefansdottir L., Juliusson K., Agustsdottir A. B., Zink F., Halldorsson G. H., Ivarsdottir E. V., Benonisdottir S., Jonsson H., Gylfason A., Norland K., Trajanoska K., Boer C. G., Southam L., Leung J. C. S., Tang N. L. S., Kwok T. C. Y., Lee J. S. W., Ho S. C., Byrjalsen I., Center J. R., Lee S. H., Koh J. M., Lohmander L. S., Ho-Pham L. T., Nguyen T. V., Eisman J. A., Woo J., Leung P. C., Loughlin J., Zeggini E., Christiansen C., Rivadeneira F., van Meurs J., Uitterlinden A. G., Mogensen B., Jonsson H., Ingvarsson T., Sigurdsson G., Benediktsson R., Sulem P., Jonsdottir I., Masson G., Holm H., Norddahl G. L., Thorsteinsdottir U., Gudbjartsson D. F., and Stefansson K. (2019) GWAS of bone size yields twelve loci that also affect height, BMD, osteoarthritis or fractures. Nature communications 10, 2054 - PMC - PubMed
-
- Chan Y., Salem R. M., Hsu Y. H., McMahon G., Pers T. H., Vedantam S., Esko T., Guo M. H., Lim E. T., Consortium G., Franke L., Smith G. D., Strachan D. P., and Hirschhorn J. N. (2015) Genome-wide analysis of body proportion classifies height-associated variants by mechanism of action and implicates genes important for skeletal development. Am J Hum Genet 96, 695–708 - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
