This is a preprint.
Fucosylated glycoproteins and fucosylated glycolipids play opposing roles in cholera intoxication
- PMID: 37577488
- PMCID: PMC10418270
- DOI: 10.1101/2023.08.02.551727
Fucosylated glycoproteins and fucosylated glycolipids play opposing roles in cholera intoxication
Update in
-
Fucosylation of glycoproteins and glycolipids: opposing roles in cholera intoxication.Nat Chem Biol. 2025 Apr;21(4):555-566. doi: 10.1038/s41589-024-01748-5. Epub 2024 Oct 16. Nat Chem Biol. 2025. PMID: 39414978 Free PMC article.
Abstract
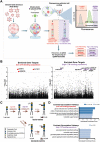
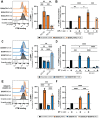
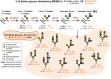
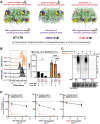
Cholera toxin (CT) is the etiological agent of cholera. Here we report that multiple classes of fucosylated glycoconjugates function in CT binding and intoxication of intestinal epithelial cells. In Colo205 cells, knockout of B3GNT5, the enzyme required for synthesis of lacto- and neolacto-series glycosphingolipids (GSLs), reduces CT binding but sensitizes cells to intoxication. Overexpressing B3GNT5 to generate more fucosylated GSLs confers protection against intoxication, indicating that fucosylated GSLs act as decoy receptors for CT. Knockout (KO) of B3GALT5 causes increased production of fucosylated O-linked and N-linked glycoproteins, and leads to increased CT binding and intoxication. Knockout of B3GNT5 in B3GALT5 KO cells eliminates production of fucosylated GSLs but increases intoxication, identifying fucosylated glycoproteins as functional receptors for CT. These findings provide insight into molecular determinants regulating CT sensitivity of host cells.
Figures

Similar articles
-
Fucosylation of glycoproteins and glycolipids: opposing roles in cholera intoxication.Nat Chem Biol. 2025 Apr;21(4):555-566. doi: 10.1038/s41589-024-01748-5. Epub 2024 Oct 16. Nat Chem Biol. 2025. PMID: 39414978 Free PMC article.
-
Cholera intoxication of human enteroids reveals interplay between decoy and functional glycoconjugate ligands.Glycobiology. 2023 Oct 30;33(10):801-816. doi: 10.1093/glycob/cwad069. Glycobiology. 2023. PMID: 37622990 Free PMC article.
-
Lack of lacto/neolacto-glycolipids enhances the formation of glycolipid-enriched microdomains, facilitating B cell activation.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11900-5. doi: 10.1073/pnas.0914298107. Epub 2010 Jun 14. Proc Natl Acad Sci U S A. 2010. PMID: 20547865 Free PMC article.
-
[The significance of fucosylated glycoconjugates of human milk in nutrition of newborns and infants].Postepy Hig Med Dosw (Online). 2015 Jul 22;69:811-29. doi: 10.5604/17322693.1162561. Postepy Hig Med Dosw (Online). 2015. PMID: 26206995 Review. Polish.
-
Structure and function of glycosphingolipids on small extracellular vesicles.Glycoconj J. 2022 Apr;39(2):197-205. doi: 10.1007/s10719-022-10052-0. Epub 2022 Feb 24. Glycoconj J. 2022. PMID: 35201531 Free PMC article. Review.
References
-
- Grant T.A., Balasubramanian D. & Almagro-Moreno S. JMM Profile: Vibrio cholerae: an opportunist of human crises. J Med Microbiol 70 (2021). - PubMed
-
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry 12, 3558–3566 (1973). - PubMed
-
- Cuatrecasas P., Parikh I. & Hollenberg M.D. Affinity chromatography and structural analysis of Vibrio cholerae enterotoxin-ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. Biochemistry 12, 4253–4264 (1973). - PubMed
-
- Heyningen S.V. Cholera toxin: interaction of subunits with ganglioside GM1. Science 183, 656–657 (1974). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
