This is a preprint.
TWISP: A Transgenic Worm for Interrogating Signal Propagation in C. elegans
- PMID: 37577580
- PMCID: PMC10418184
- DOI: 10.1101/2023.08.03.551820
TWISP: A Transgenic Worm for Interrogating Signal Propagation in C. elegans
Update in
-
TWISP: a transgenic worm for interrogating signal propagation in Caenorhabditis elegans.Genetics. 2024 Jul 8;227(3):iyae077. doi: 10.1093/genetics/iyae077. Genetics. 2024. PMID: 38733622 Free PMC article.
Abstract
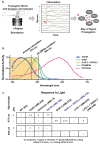
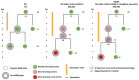
Genetically encoded optical indicators and actuators of neural activity allow for all-optical investigations of signaling in the nervous system. But commonly used indicators, actuators and expression strategies are poorly suited for systematic measurements of signal propagation at brain scale and cellular resolution. Large scale measurements of the brain require indicators and actuators with compatible excitation spectra to avoid optical crosstalk. They must be highly expressed in every neuron but at the same time avoid lethality and permit the animal to reach adulthood. And finally, their expression must be compatible with additional fluorescent labels to locate and identify neurons, such as those in the NeuroPAL cell identification system. We present TWISP, a Transgenic Worm for Interrogating Signal Propagation, that address these needs and enables optical measurements of evoked calcium activity at brain scale and cellular resolution in the nervous system of the nematode Caenorhabditis elegans. We express in every neuron a non-conventional optical actuator, the gustatory receptor homolog GUR-3+PRDX-2 under the control of a drug-inducible system QF+hGR, and calcium indicator GCAMP6s, in a background with additional fluorophores of the NeuroPAL cell ID system. We show that this combination, but not others tested, avoids optical-crosstalk, creates strong expression in the adult, and generates stable transgenic lines for systematic measurements of signal propagation in the worm brain.
Keywords: C. elegans; Dexamethasone; GUR-3; PRDX-2; calcium imaging; drug-inducible gene expression; functional connectivity; neurons; optogenetics.
Conflict of interest statement
Conflict of Interest: No conflict of interest.
Figures





References
-
- Beets I., Zels S., Vandewyer E., Demeulemeester J., Caers J. et al. , 2022. System-wide mapping of neuropeptide-GPCR interactions in C. elegans. bioRxiv: 2022.2010.2030.514428.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
