This is a preprint.
The cell adhesion molecule Echinoid promotes tissue survival and separately restricts tissue overgrowth in Drosophila imaginal discs
- PMID: 37577631
- PMCID: PMC10418178
- DOI: 10.1101/2023.08.04.552072
The cell adhesion molecule Echinoid promotes tissue survival and separately restricts tissue overgrowth in Drosophila imaginal discs
Update in
-
The cell-adhesion molecule Echinoid promotes tissue survival and separately restricts tissue overgrowth.Development. 2025 Aug 1;152(15):dev204572. doi: 10.1242/dev.204572. Epub 2025 Aug 7. Development. 2025. PMID: 40689564 Free PMC article.
Abstract
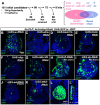
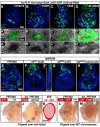
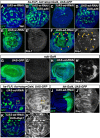
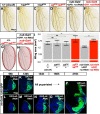
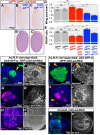
The interactions that cells in Drosophila imaginal discs have with their neighbors are known to regulate their ability to survive. In a screen of genes encoding cell surface proteins for gene knockdowns that affect the size or shape of mutant clones, we found that clones of cells with reduced levels of echinoid (ed) are fewer, smaller, and can be eliminated during development. In contrast, discs composed mostly of ed mutant tissue are overgrown. We find that ed mutant tissue has lower levels of the anti-apoptotic protein Diap1 and has increased levels of apoptosis which is consistent with the observed underrepresentation of ed mutant clones and the slow growth of ed mutant tissue. The eventual overgrowth of ed mutant tissue results not from accelerated growth, but from prolonged growth resulting from a failure to arrest growth at the appropriate final size. Ed has previously been shown to physically interact with multiple Hippo-pathway components and it has been proposed to promote Hippo pathway signaling, to exclude Yorkie (Yki) from the nucleus, and restrain the expression of Yki-target genes. We did not observe changes in Yki localization in ed mutant tissue and found decreased levels of expression of several Yorkie-target genes, findings inconsistent with the proposed effect of Ed on Yki. We did, however, observe increased expression of several Yki-target genes in wild-type cells neighboring ed mutant cells, which may contribute to elimination of ed mutant clones. Thus, ed has two distinct functions: an anti-apoptotic function by maintaining Diap1 levels, and a function to arrest growth at the appropriate final size. Both of these are unlikely to be explained by a simple effect on the Hippo pathway.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
