Viral Vectors Expressing Interleukin 2 for Cancer Immunotherapy
- PMID: 37578106
- PMCID: PMC10623065
- DOI: 10.1089/hum.2023.099
Viral Vectors Expressing Interleukin 2 for Cancer Immunotherapy
Abstract
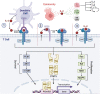
Interleukin 2 (IL-2) plays a crucial role in T cell growth and survival, enhancing the cytotoxic activity of natural killer and cytotoxic T cells and thus functioning as a versatile master proinflammatory anticancer cytokine. The FDA has approved IL-2 cytokine therapy for the treatment of metastatic melanoma and metastatic renal cell carcinoma. However, IL-2 therapy has significant constraints, including a short serum half-life, low tumor accumulation, and life-threatening toxicities associated with high doses. Oncolytic viruses (OVs) offer a promising option for cancer immunotherapy, selectively targeting and destroying cancer cells while sparing healthy cells. Numerous studies have demonstrated the successful delivery of IL-2 to the tumor microenvironment without compromising safety using OVs such as vaccinia, Sendai, parvo, Newcastle disease, tanapox, and adenoviruses. Additionally, by engineering OVs to coexpress IL-2 with other anticancer transgenes, the immune properties of IL-2 can be further enhanced. Preclinical and clinical studies have shown promising antitumor effects of IL-2-expressing viral vectors, either alone or in combination with other anticancer therapies. This review summarizes the therapeutic potential of IL-2-expressing viral vectors and their antitumor mechanisms of action.
Keywords: cancer; immunotherapy; interleukin 2; local delivery; oncolytic virus; proinflammatory cytokine; viral vectors; virotherapy.
Conflict of interest statement
S.D.R. is a co-inventor on patents relating to oncolytic herpes simplex viruses, owned and managed by Georgetown University and Massachusetts General Hospital, which have received royalties from Amgen and ActiVec, Inc., and acted as a consultant and received honoraria from Replimune, Cellinta, and Greenfire Bio, and honoraria and equity from EG 427. H.L.K. is an employee of Ankyra Therapeutics and has received honoraria for participating on advisory boards for Castle Biosciences, Midatech Pharma, Marengo Therapeutics, and Virogin. A.H. is a shareholder in Targovax ASA and an employee and a shareholder of TILT Biotherapeutics Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation.Theranostics. 2021 May 3;11(14):6668-6681. doi: 10.7150/thno.56494. eCollection 2021. Theranostics. 2021. PMID: 34093846 Free PMC article.
-
Genetically engineered Newcastle disease virus expressing interleukin-2 and TNF-related apoptosis-inducing ligand for cancer therapy.Cancer Biol Ther. 2014 Sep;15(9):1226-38. doi: 10.4161/cbt.29686. Epub 2014 Jun 27. Cancer Biol Ther. 2014. PMID: 24971746 Free PMC article.
-
Oncolytic Virotherapy: A New Paradigm in Cancer Immunotherapy.Int J Mol Sci. 2024 Jan 18;25(2):1180. doi: 10.3390/ijms25021180. Int J Mol Sci. 2024. PMID: 38256250 Free PMC article. Review.
-
New hopes for the breast cancer treatment: perspectives on the oncolytic virus therapy.Front Immunol. 2024 Mar 21;15:1375433. doi: 10.3389/fimmu.2024.1375433. eCollection 2024. Front Immunol. 2024. PMID: 38576614 Free PMC article. Review.
-
Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression.J Immunother Cancer. 2022 Mar;10(3):e003923. doi: 10.1136/jitc-2021-003923. J Immunother Cancer. 2022. PMID: 35246474 Free PMC article.
Cited by
-
An oncolytic adenovirus co-expressing a bi-specific T cell engager and IL-2 for the treatment of ovarian cancer.Mol Ther. 2024 Sep 4;32(9):2810-2813. doi: 10.1016/j.ymthe.2024.08.007. Epub 2024 Aug 23. Mol Ther. 2024. PMID: 39178849 No abstract available.
-
IL-2 based cancer immunotherapies: an evolving paradigm.Front Immunol. 2024 Jul 24;15:1433989. doi: 10.3389/fimmu.2024.1433989. eCollection 2024. Front Immunol. 2024. PMID: 39114660 Free PMC article. Review.
-
The investigation of oncolytic viruses in the field of cancer therapy.Front Oncol. 2024 Jul 10;14:1423143. doi: 10.3389/fonc.2024.1423143. eCollection 2024. Front Oncol. 2024. PMID: 39055561 Free PMC article. Review.
-
Mathematical and Machine Learning Models of Renal Cell Carcinoma: A Review.Bioengineering (Basel). 2023 Nov 16;10(11):1320. doi: 10.3390/bioengineering10111320. Bioengineering (Basel). 2023. PMID: 38002445 Free PMC article. Review.
-
New horizons in B-cell lymphoma immunotherapy: From immune checkpoints to precision medicine.Neoplasia. 2025 Sep;67:101206. doi: 10.1016/j.neo.2025.101206. Epub 2025 Jul 1. Neoplasia. 2025. PMID: 40592237 Free PMC article. Review.
References
-
- Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine 2004;28:109–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

