Assessing the Biocompatibility and Regeneration of Electrospun-Nanofiber Composite Tracheal Grafts
- PMID: 37578209
- PMCID: PMC10864676
- DOI: 10.1002/lary.30955
Assessing the Biocompatibility and Regeneration of Electrospun-Nanofiber Composite Tracheal Grafts
Abstract
Objective: Composite tracheal grafts (CTG) combining decellularized scaffolds with external biomaterial support have been shown to support host-derived neotissue formation. In this study, we examine the biocompatibility, graft epithelialization, vascularization, and patency of three prototype CTG using a mouse microsurgical model.
Study design: Tracheal replacement, regenerative medicine, biocompatible airway splints, animal model.
Method: CTG electrospun splints made by combining partially decellularized tracheal grafts (PDTG) with polyglycolic acid (PGA), poly(lactide-co-ε-caprolactone) (PLCL), or PLCL/PGA were orthotopically implanted in mice (N = 10/group). Tracheas were explanted two weeks post-implantation. Micro-Computed Tomography was conducted to assess for graft patency, and histological analysis was used to assess for epithelialization and neovascularization.
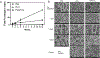
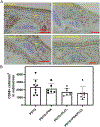
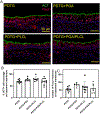
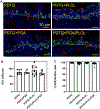
Result: Most animals (greater than 80%) survived until the planned endpoint and did not exhibit respiratory symptoms. MicroCT confirmed the preservation of graft patency. Grossly, the PDTG component of CTG remained intact. Examining the electrospun component of CTG, PGA degraded significantly, while PLCL+PDTG and PLCL/PGA + PDTG maintained their structure. Microvasculature was observed across the surface of CTG and infiltrating the pores. There were no signs of excessive cellular infiltration or encapsulation. Graft microvasculature and epithelium appear similar in all groups, suggesting that CTG did not hinder endothelialization and epithelialization.
Conclusion: We found that all electrospun nanofiber CTGs are biocompatible and did not affect graft patency, endothelialization and epithelialization. Future directions will explore methods to accelerate graft regeneration of CTG.
Level of evidence: N/A Laryngoscope, 134:1155-1162, 2024.
Keywords: Tracheal replacement; animal model; biocompatible splint; regenerative medicine.
© 2023 The American Laryngological, Rhinological and Otological Society, Inc.
Conflict of interest statement
Conflicts of Interest: None
Figures




References
-
- Tracheal disease. Tracheal Disease ∣ Michigan Medicine. (n.d.). Retrieved January 9, 2022, from https://www.uofmhealth.org/conditions-treatments/surgery/tracheal-disease
-
- Delaere P, Vranckx J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. New England Journal of Medicine 2010; 362: 138–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

