A Comparison of Treatment Effect Sizes in Matched Phase 2 and Phase 3 Trials of Advanced Therapeutics in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis
- PMID: 37578211
- PMCID: PMC10684248
- DOI: 10.14309/ctg.0000000000000629
A Comparison of Treatment Effect Sizes in Matched Phase 2 and Phase 3 Trials of Advanced Therapeutics in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis
Abstract
Introduction: Phase 2 trials are fundamental to the rational and efficient design of phase 3 trials. We aimed to determine the relationship of treatment effect size estimates from phase 2 and phase 3 clinical trials on advanced therapeutics in inflammatory bowel disease.
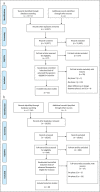
Methods: MEDLINE, EMBASE, CENTRAL, and the Cochrane library were searched from inception to December 19, 2022, to identify paired phase 2 and 3 placebo-controlled induction studies of advanced therapeutics for Crohn's disease (CD) and ulcerative colitis (UC). Treatment effect sizes were expressed as a risk ratio (RR) between the active arm and placebo arm. For the same therapeutics, RRs from phase 2 trials were divided by the RR from phase 3 trial to quantify the relationship of effect sizes between phases.
Results: Twenty-two studies (9 phase 2 trials, 13 phase 3 trials) were included for CD and 30 studies (12 phase 2 trials, 18 phase 3 trials) for UC. In UC (pooled RR 0.72; 95% confidence interval: 0.58-0.86; RR <1 indicates smaller treatment effect sizes in phase 2 trials), but not CD (pooled RR 1.01; 95% confidence interval: 0.84-1.18), phase 2 trials systematically underestimated treatment effect sizes for the primary endpoint compared with phase 3 trials. The underestimation was observed for clinical, but not endoscopic, endpoints in UC.
Discussion: Treatment effect sizes for the primary and clinical endpoints were similar across clinical trial phases in CD, but not UC, where only endoscopic endpoints were comparable. This will help inform clinical development plans and future trial design.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures

References
-
- Sedgwick P. What are the four phases of clinical research trials? BMJ 2014;348(1):g3727. - PubMed
-
- Robuck PR, Wurzelmann JI. Understanding the drug development process. Inflamm Bowel Dis 2005;11(Suppl 1):S13–6. - PubMed
-
- Harris MS, Wichary J, Zadnik M, et al. Competition for clinical trials in inflammatory bowel diseases. Gastroenterology 2019;157(6):1457–61.e2. - PubMed
-
- Morgan P, Van Der Graaf PH, Arrowsmith J, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov Today 2012;17(9-10):419–24. - PubMed
-
- Halpern SD, Karlawish JH, Berlin JA. The continuing unethical conduct of underpowered clinical trials. JAMA 2002;288(3):358–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

