Reverse genetics of parrot bornavirus 4 reveals a unique splicing of the glycoprotein gene that affects viral propagation
- PMID: 37578232
- PMCID: PMC10506466
- DOI: 10.1128/jvi.00509-23
Reverse genetics of parrot bornavirus 4 reveals a unique splicing of the glycoprotein gene that affects viral propagation
Abstract
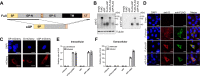
Viruses can utilize host splicing machinery to enable the expression of multiple genes from a limited-sized genome. Orthobornaviruses use alternative splicing to regulate the expression level of viral proteins and achieve efficient viral replication in the nucleus. Although more than 20 orthobornaviruses have been identified belonging to eight different viral species, virus-specific splicing has not been demonstrated. Here, we demonstrate that the glycoprotein (G) transcript of parrot bornavirus 4 (PaBV-4; species Orthobornavirus alphapsittaciforme), a highly virulent virus in psittacines, undergoes mRNA splicing and expresses a soluble isoform termed sGP. Interestingly, the splicing donor for sGP is not conserved in other orthobornaviruses, including those belonging to the same orthobornavirus species, suggesting that this splicing has evolved as a PaBV-4-specific event. We have also shown that exogenous expression of sGP does not affect PaBV-4 replication or de novo virion infectivity. In this study, to investigate the role of sGP in viral replication, we established a reverse genetics system for PaBV-4 by using avian cell lines and generated a recombinant virus lacking the spliced mRNA for sGP. Using the recombinant viruses, we show that the replication of the sGP-deficient virus is significantly slower than that of the wild-type virus and that the exogenous expression of sGP cannot restore its propagation efficiency. These results suggest that autologous or controlled expression of sGP by splicing may be important for PaBV-4 propagation. The reverse genetics system for avian bornaviruses developed here will be a powerful tool for understanding the replication strategies and pathogenesis of avian orthobornaviruses. IMPORTANCE Parrot bornavirus 4 (PaBV-4) is the dominant cause of proventricular dilatation disease, a severe gastrointestinal and central nervous system disease among avian bornaviruses. In this study, we discovered that PaBV-4 expresses a soluble isoform of glycoprotein (G), called sGP, through alternative splicing of the G mRNA, which is unique to this virus. To understand the role of sGP in viral replication, we generated recombinant PaBV-4 lacking the newly identified splicing donor site for sGP using a reverse genetics system and found that its propagation was significantly slower than that of the wild-type virus, suggesting that sGP plays an essential role in PaBV-4 infection. Our results provide important insights not only into the replication strategy but also into the pathogenesis of PaBV-4, which is the most prevalent bornavirus in captive psittacines worldwide.
Keywords: RNA splicing; avian virus; bornavirus; proventricular dilatation disease; reverse genetic analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kistler AL, Gancz A, Clubb S, Skewes-Cox P, Fischer K, Sorber K, Chiu CY, Lublin A, Mechani S, Farnoushi Y, Greninger A, Wen CC, Karlene SB, Ganem D, DeRisi JL. 2008. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J 5:88. doi: 10.1186/1743-422X-5-88 - DOI - PMC - PubMed
-
- Honkavuori KS, Shivaprasad HL, Williams BL, Quan PL, Hornig M, Street C, Palacios G, Hutchison SK, Franca M, Egholm M, Briese T, Lipkin WI. 2008. Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg Infect Dis 14:1883–1886. doi: 10.3201/eid1412.080984 - DOI - PMC - PubMed
-
- Heffels-Redmann U, Enderlein D, Herzog S, Herden C, Piepenbring A, Neumann D, Müller H, Capelli S, Müller H, Oberhäuser K, Gerlach H, Kaleta EF, Lierz M. 2011. Occurrence of avian bornavirus infection in captive psittacines in various European countries and its association with proventricular dilatation disease. Avian Pathol 40:419–426. doi: 10.1080/03079457.2011.589825 - DOI - PubMed
-
- Weissenböck H, Bakonyi T, Sekulin K, Ehrensperger F, Doneley RJT, Dürrwald R, Hoop R, Erdélyi K, Gál J, Kolodziejek J, Nowotny N. 2009. Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease. Emerg Infect Dis 15:1453–1459. doi: 10.3201/eid1509.090353 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

