A single dominant locus restricts retrovirus replication in YBR/Ei mice
- PMID: 37578238
- PMCID: PMC10506465
- DOI: 10.1128/jvi.00685-23
A single dominant locus restricts retrovirus replication in YBR/Ei mice
Abstract
Differential responses to viral infections are influenced by the genetic makeup of the host. Studies of resistance to retroviruses in human populations are complicated due to the inability to conduct proof-of-principle studies. Inbred mouse lines, which have a range of susceptible phenotypes to retroviruses, are an ideal tool to identify and characterize mechanisms of resistance and define their genetic underpinnings. YBR/Ei mice become infected with Mouse Mammary Tumor Virus, a mucosally transmitted murine retrovirus, but eliminate the virus from their pedigrees. Virus elimination correlates with a lack of virus-specific neonatal oral tolerance, which is a major mechanism for blocking the anti-virus response in susceptible mice. Virus control is unrelated to virus-neutralizing antibodies, cytotoxic CD8+ T cells, NK cells, and NK T cells, which are the best characterized mechanisms of resistance to retroviruses. We identified a single, dominant locus that controls the resistance mechanism, which we provisionally named attenuation of virus titers (Avt) and mapped to the distal region of chromosome 18. IMPORTANCE Elucidation of the mechanism that mediates resistance to retroviruses is of fundamental importance to human health, as it will ultimately lead to knowledge of the genetic differences among individuals in susceptibility to microbial infections.
Keywords: genetic mechanism; immune response; retroviruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


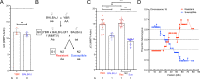
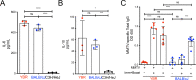
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

