Cytoarchitectonic, receptor distribution and functional connectivity analyses of the macaque frontal lobe
- PMID: 37578332
- PMCID: PMC10425179
- DOI: 10.7554/eLife.82850
Cytoarchitectonic, receptor distribution and functional connectivity analyses of the macaque frontal lobe
Abstract
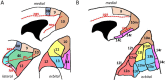
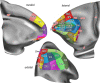
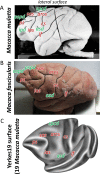
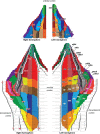
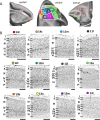
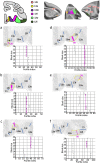
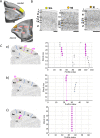
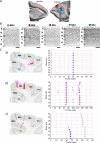
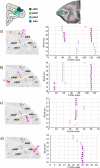
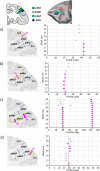
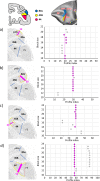
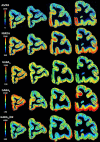
Based on quantitative cyto- and receptor architectonic analyses, we identified 35 prefrontal areas, including novel subdivisions of Walker's areas 10, 9, 8B, and 46. Statistical analysis of receptor densities revealed regional differences in lateral and ventrolateral prefrontal cortex. Indeed, structural and functional organization of subdivisions encompassing areas 46 and 12 demonstrated significant differences in the interareal levels of α2 receptors. Furthermore, multivariate analysis included receptor fingerprints of previously identified 16 motor areas in the same macaque brains and revealed 5 clusters encompassing frontal lobe areas. We used the MRI datasets from the non-human primate data sharing consortium PRIME-DE to perform functional connectivity analyses using the resulting frontal maps as seed regions. In general, rostrally located frontal areas were characterized by bigger fingerprints, that is, higher receptor densities, and stronger regional interconnections. Whereas more caudal areas had smaller fingerprints, but showed a widespread connectivity pattern with distant cortical regions. Taken together, this study provides a comprehensive insight into the molecular structure underlying the functional organization of the cortex and, thus, reconcile the discrepancies between the structural and functional hierarchical organization of the primate frontal lobe. Finally, our data are publicly available via the EBRAINS and BALSA repositories for the entire scientific community.
Keywords: cytoarchitecture; functional connectivity; macaque; motor; neuroscience; prefrontal; receptor; rhesus macaque.
© 2023, Rapan et al.
Conflict of interest statement
LR, SF, MN, TX, LZ, TF, XW, KA, NP No competing interests declared
Figures






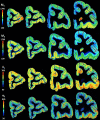
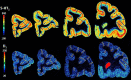


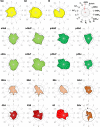

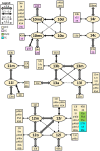
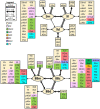
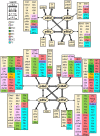
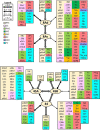
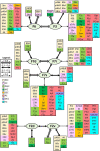
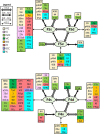
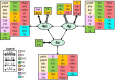
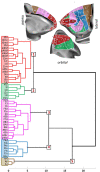
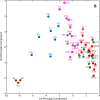
Update of
- doi: 10.1101/2022.06.27.497757
Similar articles
-
Multimodal 3D atlas of the macaque monkey motor and premotor cortex.Neuroimage. 2021 Feb 1;226:117574. doi: 10.1016/j.neuroimage.2020.117574. Epub 2020 Nov 20. Neuroimage. 2021. PMID: 33221453 Free PMC article.
-
Connectivity-based parcellation of the macaque frontal cortex, and its relation with the cytoarchitectonic distribution described in current atlases.Brain Struct Funct. 2017 Apr;222(3):1331-1349. doi: 10.1007/s00429-016-1280-3. Epub 2016 Jul 28. Brain Struct Funct. 2017. PMID: 27469273
-
Medial to lateral frontal functional connectivity mapping reveals the organization of cingulate cortex.Cereb Cortex. 2024 Aug 1;34(8):bhae322. doi: 10.1093/cercor/bhae322. Cereb Cortex. 2024. PMID: 39129533
-
The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains.Cortex. 2012 Jan;48(1):46-57. doi: 10.1016/j.cortex.2011.07.002. Epub 2011 Jul 29. Cortex. 2012. PMID: 21872854 Review.
-
Lateral prefrontal cortex: architectonic and functional organization.Philos Trans R Soc Lond B Biol Sci. 2005 Apr 29;360(1456):781-95. doi: 10.1098/rstb.2005.1631. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 15937012 Free PMC article. Review.
Cited by
-
Decision-making shapes dynamic inter-areal communication within macaque ventral frontal cortex.Curr Biol. 2024 Oct 7;34(19):4526-4538.e5. doi: 10.1016/j.cub.2024.08.034. Epub 2024 Sep 17. Curr Biol. 2024. PMID: 39293441
-
Dissociable Representations of Decision Variables within Subdivisions of the Macaque Orbital and Ventrolateral Frontal Cortex.J Neurosci. 2024 Aug 28;44(35):e0464242024. doi: 10.1523/JNEUROSCI.0464-24.2024. J Neurosci. 2024. PMID: 38991790 Free PMC article.
-
Proteomic features of gray matter layers and superficial white matter of the rhesus monkey neocortex: comparison of prefrontal area 46 and occipital area 17.Brain Struct Funct. 2024 Sep;229(7):1495-1525. doi: 10.1007/s00429-024-02819-y. Epub 2024 Jun 28. Brain Struct Funct. 2024. PMID: 38943018 Free PMC article.
-
Monkey Lateral Prefrontal Cortex Subregions Differentiate between Perceptual Exposure to Visual Stimuli.J Cogn Neurosci. 2025 Apr 1;37(4):802-814. doi: 10.1162/jocn_a_02291. J Cogn Neurosci. 2025. PMID: 39785668 Free PMC article.
-
Dissociable representations of decision variables within subdivisions of macaque orbitofrontal and ventrolateral frontal cortex.bioRxiv [Preprint]. 2024 Mar 13:2024.03.10.584181. doi: 10.1101/2024.03.10.584181. bioRxiv. 2024. Update in: J Neurosci. 2024 Aug 28;44(35):e0464242024. doi: 10.1523/JNEUROSCI.0464-24.2024. PMID: 38559221 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

