Normal and dysregulated crosstalk between iron metabolism and erythropoiesis
- PMID: 37578340
- PMCID: PMC10425177
- DOI: 10.7554/eLife.90189
Normal and dysregulated crosstalk between iron metabolism and erythropoiesis
Abstract
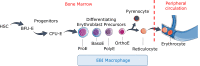
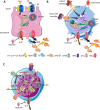
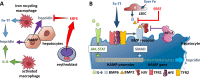
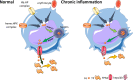
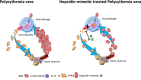
Erythroblasts possess unique characteristics as they undergo differentiation from hematopoietic stem cells. During terminal erythropoiesis, these cells incorporate large amounts of iron in order to generate hemoglobin and ultimately undergo enucleation to become mature red blood cells, ultimately delivering oxygen in the circulation. Thus, erythropoiesis is a finely tuned, multifaceted process requiring numerous properly timed physiological events to maintain efficient production of 2 million red blood cells per second in steady state. Iron is required for normal functioning in all human cells, the erythropoietic compartment consuming the majority in light of the high iron requirements for hemoglobin synthesis. Recent evidence regarding the crosstalk between erythropoiesis and iron metabolism sheds light on the regulation of iron availability by erythroblasts and the consequences of insufficient as well as excess iron on erythroid lineage proliferation and differentiation. In addition, significant progress has been made in our understanding of dysregulated iron metabolism in various congenital and acquired malignant and non-malignant diseases. Finally, we report several actual as well as theoretical opportunities for translating the recently acquired robust mechanistic understanding of iron metabolism regulation to improve management of patients with disordered erythropoiesis, such as anemia of chronic inflammation, β-thalassemia, polycythemia vera, and myelodysplastic syndromes.
Keywords: anemia; developmental biology; erythropoiesis; iron deficiency; medicine; polycythemia.
© 2023, Ginzburg et al.
Conflict of interest statement
YG Reviewing editor, eLife, XA, SR, AG No competing interests declared
Figures
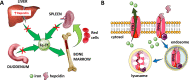

References
-
- Adema V, Ma F, Kanagal-Shamanna R, Thongon N, Montalban-Bravo G, Yang H, Peslak SA, Wang F, Acha P, Sole F, Lockyer P, Cassari M, Maciejewski JP, Visconte V, Gañán-Gómez I, Song Y, Bueso-Ramos C, Pellegrini M, Tan TM, Bejar R, Carew JS, Halene S, Santini V, Al-Atrash G, Clise-Dwyer K, Garcia-Manero G, Blobel GA, Colla S. Targeting the EIF2AK1 signaling pathway rescues red blood cell production in SF3B1-mutant myelodysplastic syndromes with ringed sideroblasts. Blood Cancer Discovery. 2022;3:554–567. doi: 10.1158/2643-3230.BCD-21-0220. - DOI - PMC - PubMed
-
- Ali AM, Huang Y, Pinheiro RF, Xue F, Hu J, Iverson N, Hoehn D, Coutinho D, Kayani J, Chernak B, Lane J, Hillyer C, Galili N, Jurcic J, Mohandas N, An X, Raza A. Severely impaired terminal erythroid differentiation as an independent prognostic marker in myelodysplastic syndromes. Blood Advances. 2018;2:1393–1402. doi: 10.1182/bloodadvances.2018018440. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL140625/HL/NHLBI NIH HHS/United States
- DK133475/NH/NIH HHS/United States
- DK079924/NH/NIH HHS/United States
- R56 DK132146/DK/NIDDK NIH HHS/United States
- DK107670/NH/NIH HHS/United States
- DK101550/NH/NIH HHS/United States
- R01 DK107670/DK/NIDDK NIH HHS/United States
- DK132146/NH/NIH HHS/United States
- R01 HL165202/HL/NHLBI NIH HHS/United States
- R01 DK079924/DK/NIDDK NIH HHS/United States
- HL149626/NH/NIH HHS/United States
- R01 DK133475/DK/NIDDK NIH HHS/United States
- P01 HL149626/HL/NHLBI NIH HHS/United States
- R56 HL165202/HL/NHLBI NIH HHS/United States
- R01 DK095112/DK/NIDDK NIH HHS/United States
- HL140625/NH/NIH HHS/United States
- HL165202/NH/NIH HHS/United States
- DK095112/NH/NIH HHS/United States
- R01 DK101550/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

