Neuronal tau pathology worsens late-phase white matter degeneration after traumatic brain injury in transgenic mice
- PMID: 37578550
- PMCID: PMC10499978
- DOI: 10.1007/s00401-023-02622-9
Neuronal tau pathology worsens late-phase white matter degeneration after traumatic brain injury in transgenic mice
Abstract
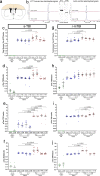
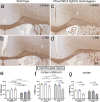
Traumatic brain injury (TBI) causes diffuse axonal injury which can produce chronic white matter pathology and subsequent post-traumatic neurodegeneration with poor patient outcomes. Tau modulates axon cytoskeletal functions and undergoes phosphorylation and mis-localization in neurodegenerative disorders. The effects of tau pathology on neurodegeneration after TBI are unclear. We used mice with neuronal expression of human mutant tau to examine effects of pathological tau on white matter pathology after TBI. Adult male and female hTau.P301S (Tg2541) transgenic and wild-type (Wt) mice received either moderate single TBI (s-TBI) or repetitive mild TBI (r-mTBI; once daily × 5), or sham procedures. Acutely, s-TBI produced more extensive axon damage in the corpus callosum (CC) as compared to r-mTBI. After s-TBI, significant CC thinning was present at 6 weeks and 4 months post-injury in Wt and transgenic mice, with homozygous tau expression producing additional pathology of late demyelination. In contrast, r-mTBI did not produce significant CC thinning except at the chronic time point of 4 months in homozygous mice, which exhibited significant CC atrophy (- 29.7%) with increased microgliosis. Serum neurofilament light quantification detected traumatic axonal injury at 1 day post-TBI in Wt and homozygous mice. At 4 months, high tau and neurofilament in homozygous mice implicated tau in chronic axon pathology. These findings did not have sex differences detected. Conclusions: Neuronal tau pathology differentially exacerbated CC pathology based on injury severity and chronicity. Ongoing CC atrophy from s-TBI became accompanied by late demyelination. Pathological tau significantly worsened CC atrophy during the chronic phase after r-mTBI.
Keywords: Axon damage; Demyelination; Neuroregeneration; Tau; Traumatic brain injury; White matter.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors have no conflicts of interest to disclose. The views, information or content, and conclusions presented do not necessarily represent the official position or policy of, nor should any official endorsement be inferred on the part of, the Uniformed Services University, the Department of Defense, the U.S. Government or the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
Figures











References
-
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. - DOI - PMC - PubMed
-
- Alosco ML, Stein TD, Tripodis Y, Chua AS, Kowall NW, Huber BR, Goldstein LE, Cantu RC, Katz DI, Palmisano JN, et al. Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol. 2019;76:1298–1308. doi: 10.1001/jamaneurol.2019.2244. - DOI - PMC - PubMed
-
- Bachstetter AD, Morganti JM, Bodnar CN, Webster SJ, Higgins EK, Roberts KN, Snider H, Meier SE, Nation GK, Goulding DS, et al. The effects of mild closed head injuries on tauopathy and cognitive deficits in rodents: primary results in wild type and rTg4510 mice, and a systematic review. Exp Neurol. 2020;326:113180. doi: 10.1016/j.expneurol.2020.113180. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

