Presynapses contain distinct actin nanostructures
- PMID: 37578754
- PMCID: PMC10424573
- DOI: 10.1083/jcb.202208110
Presynapses contain distinct actin nanostructures
Abstract
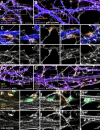
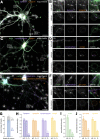
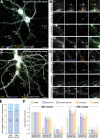
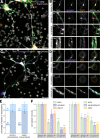
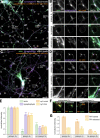
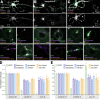
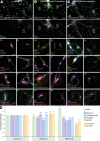
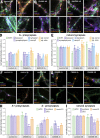
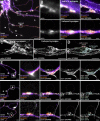
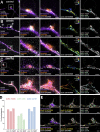
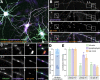
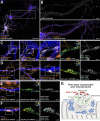
The architecture of the actin cytoskeleton that concentrates at presynapses remains poorly known, hindering our understanding of its roles in synaptic physiology. In this work, we measure and visualize presynaptic actin by diffraction-limited and super-resolution microscopy, thanks to a validated model of bead-induced presynapses in cultured neurons. We identify a major population of actin-enriched presynapses that concentrates more presynaptic components and shows higher synaptic vesicle cycling than their non-enriched counterparts. Pharmacological perturbations point to an optimal actin amount and the presence of distinct actin structures within presynapses. We directly visualize these nanostructures using Single Molecule Localization Microscopy (SMLM), defining three distinct types: an actin mesh at the active zone, actin rails between the active zone and deeper reserve pools, and actin corrals around the whole presynaptic compartment. Finally, CRISPR-tagging of endogenous actin allows us to validate our results in natural synapses between cultured neurons, confirming the role of actin enrichment and the presence of three types of presynaptic actin nanostructures.
© 2023 Bingham et al.
Conflict of interest statement
Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. K. Friedl reported “Employee of Abbelight.” No other disclosures were reported.
Figures





References
-
- Aurnhammer, C., Haase M., Muether N., Hausl M., Rauschhuber C., Huber I., Nitschko H., Busch U., Sing A., Ehrhardt A., and Baiker A.. 2012. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods. 23:18–28. 10.1089/hgtb.2011.034 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

