Association of Intensive Lifestyle Intervention for Type 2 Diabetes With Labor Market Outcomes
- PMID: 37578773
- PMCID: PMC10425863
- DOI: 10.1001/jamainternmed.2023.3283
Association of Intensive Lifestyle Intervention for Type 2 Diabetes With Labor Market Outcomes
Abstract
Importance: An intensive lifestyle intervention (ILI) has been shown to improve diabetes management and physical function. These benefits could lead to better labor market outcomes, but this has not been previously studied.
Objective: To estimate the association of an ILI for weight loss in type 2 diabetes with employment, earnings, and disability benefit receipt during and after the intervention.
Design, setting, and participants: This cohort study included participants with type 2 diabetes and overweight or obesity and compared an ILI with a control condition of diabetes support and education. Data for the original trial were accrued from August 22, 2001, to September 14, 2012. Trial data were linked with Social Security Administration records to investigate whether, relative to the control group, the ILI was associated with improvements in labor market outcomes during and after the intervention period. Difference-in-differences models estimating relative changes in employment, earnings, and disability benefit receipt between the ILI and control groups were used, accounting for prerandomization differences in outcomes for linked participants. Outcome data were analyzed from July 13, 2020, to May 17, 2023.
Exposure: The ILI consisted of sessions with lifestyle counselors, dieticians, exercise specialists, and behavioral therapists on a weekly basis in the first 6 months, decreasing to a monthly basis by the fourth year, designed to achieve and maintain at least 7% weight loss. The control group received group-based diabetes education sessions 3 times annually during the first 4 years, with 1 annual session thereafter.
Main outcomes and measures: Employment and receipt of federal disability benefits (Supplemental Security Income and Social Security Disability Insurance), earnings, and disability benefit payments from 1994 through 2018.
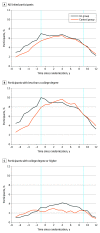
Results: A total of 3091 trial participants were linked with Social Security Administration data (60.1% of 5145 participants initially randomized and 97.0% of 3188 of participants consenting to linkage). Among the 3091 with fully linked data, 1836 (59.4%) were women, and mean (SD) age was 58.4 (6.5) years. Baseline clinical and demographic characteristics were similar between linked participants in the ILI and control groups. Employment increased by 2.9 (95% CI, 0.3-5.5) percentage points for the ILI group relative to controls (P = .03) with no significant relative change in disability benefit receipt (-0.9 [95% CI, -2.1 to 0.3] percentage points; P = .13).
Conclusions and relevance: The findings of this cohort study suggest that an ILI to prevent the progression and complications of type 2 diabetes was associated with higher levels of employment. Labor market productivity should be considered when evaluating interventions to manage chronic diseases.
Conflict of interest statement
Figures


References
-
- Community Preventive Services Task Force . Diabetes management: intensive lifestyle interventions for patients with type 2 diabetes. Updated September 5, 2017. Accessed February 2, 2023. https://www.thecommunityguide.org/findings/diabetes-intensive-lifestyle-...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

